An Optical Fiber Sensor for Uranium Detection in Water †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
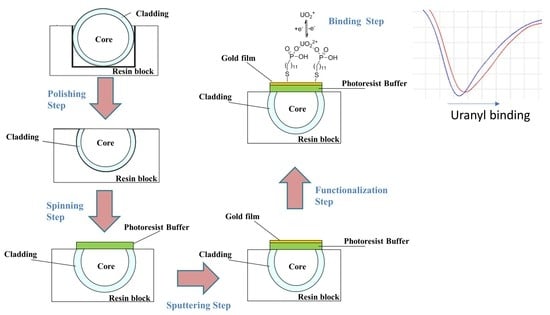
2.2. SPR-POF Platform Preparation
2.3. MUPA Deposition
2.4. Experimental Setup
2.5. Measurements
3. Results
3.1. SPR Characterization of the Sensor
3.2. Reproducibility
3.3. Sensitivity to the Refractive Index of the Aqueous Sample
3.4. Characterization of the Sensor Response to Uranyl Ion
3.5. Interferences by Ionic Components of the Sample
3.6. Response to Uranyl in Tap Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caucheteur, C.; Guo, T.; Albert, J. Review of plasmonic fiber optic biochemical sensors: Improving the limit of detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, M.; Kuncová, G.; Trögl, J. Fiber-Optic Chemical Sensors and Fiber-Optic Bio-Sensors. Sensors 2015, 15, 25208–25259. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Granville, A.M. Polymer fiber optic sensors-a mini review of their synthesis and applications. J. Biosens. Bioelectron 2016, 2016 7, 1–11. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Zeni, L. A review on simple and highly sensitive plastic optical fiber probes for bio-chemical sensing. Sens. Actuators B Chem. 2021, 331, 129393. [Google Scholar] [CrossRef]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low Cost Sensors Based on SPR in a Plastic Optical Fiber for Biosensor Implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Varriale, A.; Pennacchio, A.; Staiano, M.; Massarotti, D.; Zeni, L.; D’Auria, S. An innovative plastic optical fiber based biosensor for new bio/ applications. The case of celiac disease. Sens. Actuators B Chem. 2013, 176, 1008–1014. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Lunelli, L.; Vanzetti, L.E.; Pederzolli, C.; Zeni, L.; Pasquardini, L. An easy way to realize SPR aptasensor: A multimode plastic optical fiber platform for cancer biomarkers detection. Talanta 2015, 140, 88–95. [Google Scholar] [CrossRef]
- Cennamo, N.; Pasquardini, L.; Arcadio, F.; Vanzetti, L.E.; Bossi, A.M.; Zeni, L. D-shaped plastic optical fibre aptasensor for fast thrombin detection in nanomolar range. Sci. Rep. 2019, 9, 18740. [Google Scholar] [CrossRef]
- Cennamo, N.; Donà, A.; Pallavicini, P.; D’Agostino, G.; Dacarro, G.; Zeni, L.; Pesavento, M. Sensitive detection of 2,4,6-trinitrotoluene by tridimensional monitoring of molecularly imprinted polymer with optical fiber and five-branched gold nano stars. Sens. Actuators B Chem. 2015, 208, 291–298. [Google Scholar] [CrossRef]
- Cennamo, N.; Zeni, L.; Ricca, E.; Isticato, R.; Marzullo, V.M.; Capo, A.; Staiano, M.; D’Auria, S.; Varriale, A. Detection of naphthalene in sea-water by a label-free plasmonic optical fiber biosensor. Talanta 2019, 194, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, M.; Zeni, L.; De Maria, L.; Alberti, G.; Cennamo, N. SPR-Optical Fiber-Molecularly Imprinted Polymer Sensor for the Detection of Furfural in Wine. Biosensors 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Alberti, G.; Pesavento, M.; D’Agostino, G.; Quattrini, F.; Biesuz, R.; Zeni, L. A Simple Small Size and Low Cost Sensor Based on Surface Plasmon Resonance for Selective Detection of Fe(III). Sensors 2014, 14, 4657–4661. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, M.; Profumo, A.; Merli, D.; Cucca, L.; Zeni, L.; Cennamo, N. An Optical Fiber Chemical Sensor for the Detection of Copper(II) in Drinking Water. Sensors 2019, 19, 5246. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality 4th Edition, Incorporating the 1st Addendum. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf (accessed on 27 May 2022).
- Xiong, L.; Alshamsi, D.; Yi, P.; Hou, X.; Murad, A.; Hussein, S.; Aldahan, A.; Mohamed, M.M. Distribution of uranium isotopes in groundwater of the UAE: Environmental radioactivity assessment. J. Radioanal. Nucl. Chem. 2020, 325, 57–66. [Google Scholar] [CrossRef]
- Alberti, G.; Biesuz, R.; Pesavento, M. Determination of the total concentration and speciation of uranium in natural waters by the Resin Titration method. Microchem. J. 2007, 86, 166–173. [Google Scholar] [CrossRef]
- Merli, D.; Protti, S.; Labò, M.; Pesavento, M.; Profumo, A. A ω-mercaptoundecylphosphonic acid chemically modified gold electrode for uranium determination in waters in presence of organic matter. Talanta 2016, 151, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wang, Z.; Liu, J.; Lu, Y. Highly sensitive and selective colorimetric sensors for uranyl (UO2(2+)): Development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems. J. Am. Chem. Soc. 2008, 130, 14217–14226. [Google Scholar] [CrossRef]
- Liu, W.; Dai, X.; Bai, Z.; Wang, Y.; Yang, Z.; Zhang, L.; Xu, L.; Chen, L.; Li, Y.; Gui, D.; et al. Highly Sensitive and Selective Uranium Detection in Natural Water Systems Using a Luminescent Mesoporous Metal-Organic Framework Equipped with Abundant Lewis Basic Sites: A Combined Batch, X-ray Absorption Spectroscopy, and First Principles Simulation Investigation. Environ. Sci. Technol. 2017, 51, 3911–3921. [Google Scholar]
- Cennamo, N.; Pesavento, M.; Merli, D.; Profumo, A.; Zeni, L.; Alberti, G. An Optical Fiber Sensor System for Uranium Detection in Water. Eng. Proc. 2022, 16, 10. [Google Scholar] [CrossRef]
- García-Calzón, J.A.; Díaz-García, M.E. Characterization of binding sites in molecularly imprinted polymers. Sens. Actuators B Chem. 2007, 123, 1180–1194. [Google Scholar] [CrossRef]
- Cennamo, N.; Chiavaioli, F.; Trono, C.; Tombelli, S.; Giannetti, A.; Baldini, F.; Zeni, L. A Complete Optical Sensor System Based on a POF-SPR Platform and a Thermo-Stabilized Flow Cell for Biochemical Applications. Sensors 2016, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Kadir, N.A.A.; Irawati, N.; Jafry, A.A.A.; Razali, N.M.; Hamzah, A.; Harun, S.W. Sodium nitrate sensor based on D-shaped fiber structure. Measurement 2020, 163, 107927. [Google Scholar] [CrossRef]
- Popov, K.; Rönkkömäki, H.; Lajunen, L.H. Critical evaluation of stability constants of phosphonic acids (IUPAC technical report). Pure Appl. Chem. 2001, 73, 1641–1677. [Google Scholar] [CrossRef]
- Gonzalez-Estrella, J.; Meza, I.; Burns, A.J.; Ali, A.S.; Lezama-Pacheco, J.S.; Lichtner, P.; Shaikh, N.; Fendorf, S.; Cerrato, J.M. Effect of Bicarbonate, Calcium, and pH on the Reactivity of As(V) and U(VI) Mixtures. Environ. Sci. Technol. 2020, 54, 3979–3987. [Google Scholar] [CrossRef]
- Maher, K.; Bargar, J.R.; Brown, G.E., Jr. Environmental speciation of actinides. Inorg. Chem. 2013, 52, 3510–3532. [Google Scholar] [CrossRef]
- Crea, F.; De Robertis, A.; De Stefano, C.; Sammartano, S. Dioxouranium(VI)-carboxylate complexes. A calorimetric and potentiometric investigation of interaction with oxalate at infinite dilution and in NaCl aqueous solution at I = 1.0 mol L(−1) and T = 25 degrees C. Talanta 2007, 71, 948–963. [Google Scholar] [CrossRef]
- Austin, R.W.; Halikas, G. The Index of Refraction of Seawater. Technical Report. 1976, UC San Diego: Scripps Institution of Oceanography, CA. Available online: https://escholarship.org/uc/item/8px2019m (accessed on 13 July 2022).










| Site | Δλmax /nm | SE Δλmax /nm | Kd /mg L−1 | SE Kd /mg L−1 | Kaff /M−1 | Sens. /nm mg−1 L | LOD /mg L−1 | Adj. R2 | HL /mg L−1 |
|---|---|---|---|---|---|---|---|---|---|
| S | 2.11 | 0.37 | 0.013 | 0.004 | 1.84·107 | 162.8 | 0.007 | 0.747 | 0.011 |
| W | 7.66 | 0.31 | 4.69 | 0.32 | 5.08·104 | 1.6 | 0.44 | 0.985 | 4.6 |
| Cation | Range | Δλmax /nm | SE Δλmax /nm | Kd /mg L−1 | Kaff /M−1 | Sens. /nm mg−1 L | LOD /mg L−1 | Adj. R2 |
|---|---|---|---|---|---|---|---|---|
| Ca(II) (red shift) | saturation at 300 mg L−1 | 2.99 | 0.53 | 0.09 | 1·103 | 0.075 | 16.6 | 0.86 |
| Mg(II) (blue shift) | saturation at 10 mg L−1 | 3.93 | 0.29 | 0.39 | 1·105 | 9.95 | 0.085 | 0.96 |
| Conditions | Δλmax /nm | SE Δλmax /nm | Kd /mg L−1 | Kaff /M−1 | Sens. /nm mg−1 L | LOD /mg L−1 | Adj. R2 |
|---|---|---|---|---|---|---|---|
| NaNO3 0.1 M | 3.79 | 0.49 | 0.01 | 2.93·107 | 466.5 | 0.0025 | 0.72 |
| NaNO3 0.1 M + Mg2+ 222 mg L−1 pH = 2 | 3.31 | 0.14 | 0.09 | 2.61·106 | 36.3 | 0.007 | 0.87 |
| NaNO3 0.1 M + Ca2+ 625 mg L−1 pH = 6 | 2.99 | 0.42 | 0.025 | 1.12·107 | 118 | 0.007 | 0.66 |
| TW + NaNO3 0.1 M | 2.07 | 0.29 | 0.061 | 3.77·106 | 33.9 | 0.008 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, G.; Pesavento, M.; De Maria, L.; Cennamo, N.; Zeni, L.; Merli, D. An Optical Fiber Sensor for Uranium Detection in Water. Biosensors 2022, 12, 635. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12080635
Alberti G, Pesavento M, De Maria L, Cennamo N, Zeni L, Merli D. An Optical Fiber Sensor for Uranium Detection in Water. Biosensors. 2022; 12(8):635. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12080635
Chicago/Turabian StyleAlberti, Giancarla, Maria Pesavento, Letizia De Maria, Nunzio Cennamo, Luigi Zeni, and Daniele Merli. 2022. "An Optical Fiber Sensor for Uranium Detection in Water" Biosensors 12, no. 8: 635. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12080635