Rhamnolipid Nano-Micelles Inhibit SARS-CoV-2 Infection and Have No Dermal or Eye Toxic Effects in Rabbits
Abstract
:1. Introduction
2. Results
2.1. Production of Rhamnolipids
2.2. Preparation and Characterization of Rhamnolipids Nano-Micelles
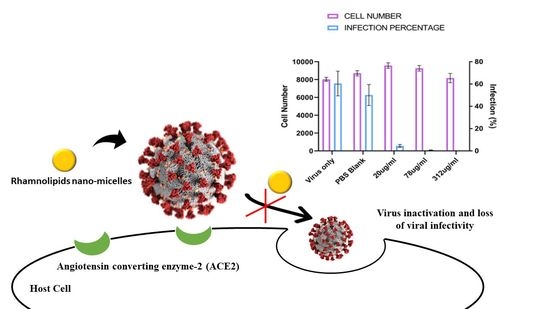
2.3. Antiviral Activity of Rhamnolipids Nano-Micelles
2.4. Irritation and Skin Sensitization Test
2.4.1. Skin Irritation Test
2.4.2. Eye Irritation Test
2.4.3. Skin Sensitization Test
2.4.4. Histopathological and Immunohistochemical Evaluations
2.5. Biochemical Analysis
2.6. Hematological Examination
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methodology
4.2.1. Production of Rhamnolipids
4.2.2. Preparation of Rhamnolipids Nano-Micelles
4.2.3. Characterization of Rhamnolipids Nano-Micelles
Particle Size and Zeta Potential
Transmission Electron Microscopy
Determination of pH
4.2.4. SARS-CoV-2 Infection Experiments (HCoV-19/England/2/202 Strain)
4.2.5. Irritation and Skin Sensitization Study
Animal
Eye Irritation Test
Skin Irritation Test
Skin Sensitization Test
Histopathological and Immunohistochemical Evaluations
Biochemical Analysis
Hematological Examination
4.2.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, M.; McKimm, J.; Sartelli, M.; Dhingra, S.; Labricciosa, F.M.; Islam, S.; Jahan, D.; Nusrat, T.; Chowdhury, T.S.; Coccolini, F.; et al. Strategies to Prevent Healthcare-Associated Infections: A Narrative Overview. Risk Manag. Healthc. Policy 2020, 13, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Garcell, H.G.; Arias, A.V.; Pancorbo Sandoval, C.A.; García, E.G.; Valle Gamboa, M.E.; Sado, A.B.; Alfonso Serrano, R.N. Incidence and Etiology of Surgical Site Infections in Appendectomies: A 3-Year Prospective Study. Oman Med. J. 2017, 32, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Al-Mousa, H.H.; Omar, A.A.; Rosenthal, V.D.; Salama, M.F.; Aly, N.Y.; El-Dossoky Noweir, M.; Rebello, F.M.; Narciso, D.M.; Sayed, A.F.; Kurian, A.; et al. Device-Associated Infection Rates, Bacterial Resistance, Length of Stay, and Mortality in Kuwait: International Nosocomial Infection Consortium Findings. Am. J. Infect. Control 2016, 44, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Ananda, T.; Modi, A.; Chakraborty, I.; Managuli, V.; Mukhopadhyay, C.; Mazumder, N. Nosocomial Infections and Role of Nanotechnology. Bioengineering 2022, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.; Buonocore, C.; Zannella, C.; Chianese, A.; Esposito, F.P.; Tedesco, P.; De Filippis, A.; Galdiero, M.; Franci, G.; de Pascale, D. Antiviral Activity of the Rhamnolipids Mixture from the Antarctic Bacterium Pseudomonas gessardii M15 against Herpes Simplex Viruses and Coronaviruses. Pharmaceutics 2021, 13, 2121. [Google Scholar] [CrossRef]
- Bakkar, M.R.; Faraag, A.H.I.; Soliman, E.R.S.; Fouda, M.S.; Sarguos, A.M.M.; McLean, G.R.; Hebishy, A.M.S.; Elkhouly, G.E.; Raya, N.R.; Abo-zeid, Y. Rhamnolipids Nano-Micelles as a Potential Hand Sanitizer. Antibiotics 2021, 10, 751. [Google Scholar] [CrossRef]
- Kratzel, A.; Kratzel, A.; Todt, D.; V’kovski, P.; Steiner, S.; Steiner, S.; Gultom, M.; Gultom, M.; Thao, T.T.N.; Thao, T.T.N.; et al. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg. Infect. Dis. 2020, 26, 1592–1595. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of Coronaviruses on Inanimate Surfaces and Their Inactivation with Biocidal Agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS Coronaviruses and Influenza Virus in Healthcare Settings: The Possible Role of Dry Surface Contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef] [Green Version]
- Pittet, D.; Allegranzi, B.; Sax, H.; Dharan, S.; Pessoa-Silva, C.L.; Donaldson, L.; Boyce, J.M. Evidence-Based Model for Hand Transmission during Patient Care and the Role of Improved Practices. Lancet Infect. Dis. 2006, 6, 641–652. [Google Scholar] [CrossRef]
- Emami, A.; Javanmardi, F.; Keshavarzi, A.; Pirbonyeh, N. Hidden Threat Lurking behind the Alcohol Sanitizers in COVID-19 Outbreak. Dermatol. Ther. 2020, 33, 17–19. [Google Scholar] [CrossRef]
- Onyedibe, K.I.; Shehu, N.Y.; Pires, D.; Isa, S.E.; Okolo, M.O.; Gomerep, S.S.; Ibrahim, C.; Igbanugo, S.J.; Odesanya, R.U.; Olayinka, A.; et al. Assessment of Hand Hygiene Facilities and Staff Compliance in a Large Tertiary Health Care Facility in Northern Nigeria: A Cross Sectional Study. Antimicrob. Resist. Infect. Control 2020, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Chiang, W.L.; Chen, T.W.; Liu, M.Y.; Hsu, C.J. Application and Robust H Control of PDC Fuzzy Controller for Nonlinear Systems with External Disturbance. J. Mar. Sci. Technol. 2001, 9, 84–90. [Google Scholar] [CrossRef]
- Slaughter, R.J.; Mason, R.W.; Beasley, D.M.G.; Vale, J.A.; Schep, L.J. Isopropanol Poisoning. Clin. Toxicol. 2014, 52, 470–478. [Google Scholar] [CrossRef]
- Vogel, L. Hand Sanitizers May Increase Norovirus Risk. CMAJ 2011, 183, 799–800. [Google Scholar] [CrossRef] [Green Version]
- Blaney, D.D.; Daly, E.R.; Kirkland, K.B.; Tongren, J.E.; Kelso, P.T.; Talbot, E.A. Use of Alcohol-Based Hand Sanitizers as a Risk Factor for Norovirus Outbreaks in Long-Term Care Facilities in Northern New England: December 2006 to March 2007. Am. J. Infect. Control 2011, 39, 296–301. [Google Scholar] [CrossRef]
- Pidot, S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R.; Carter, G.P.; Lee, J.Y.H.; Lam, M.M.C.; Grayson, M.L.; Ballard, S.A.; et al. Increasing Tolerance of Hospital Enterococcus faecium to Handwash Alcohols. Sci. Transl. Med. 2018, 10, eaar6115. [Google Scholar] [CrossRef] [Green Version]
- Hayat, A.; Munnawar, F. Antibacterial Effectiveness of Commercially Available Hand Sanitizers. Int. J. Biol. Biotech 2016, 13, 427–431. [Google Scholar]
- Mahmood, A.; Eqan, M.; Pervez, S.; Ahmed, H.; Bari, A. COVID-19 and Frequent Use of Hand Sanitizers; Human Health and Environmental Hazards by Exposure Pathways. Sci. Total Environ. 2020, 742, 140561. [Google Scholar] [CrossRef]
- Müller, M.M.; Hörmann, B.; Syldatk, C.; Hausmann, R. Pseudomonas aeruginosa PAO1 as a Model for Rhamnolipid Production in Bioreactor Systems. Appl. Microbiol. Biotechnol. 2010, 87, 167–174. [Google Scholar] [CrossRef]
- Chong, H.; Li, Q. Microbial Production of Rhamnolipids: Opportunities, Challenges and Strategies. Microb. Cell Fact. 2017, 16, 137. [Google Scholar] [CrossRef]
- Henkel, M.; Geissler, M.; Weggenmann, F.; Hausmann, R. Production of Microbial Biosurfactants: Status Quo of Rhamnolipid and Surfactin towards Large-Scale Production. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Bakkar, M.R.; Elkhouly, G.E.; Raya, N.R.; Zaafar, D. Rhamnolipid Nano-Micelles Versus Alcohol-Based Hand Sanitizer: A Comparative Study for Antibacterial Activity against Hospital-Acquired Infections and Toxicity Concerns. Antibiotics 2022, 11, 605. [Google Scholar]
- Magnusson, B.; Kligman, A.M. The Identification of Contact Allergens by Animal Assay. The Guinea Pig Maximization Test. J. Invest. Dermatol. 1969, 52, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Z.; Liang, Z.; Han, L.; Feng, H.; He, S.; Zhang, J. Fabrication of a Drug Delivery System That Enhances Antifungal Drug Corneal Penetration. Drug Deliv. 2018, 25, 938–949. [Google Scholar] [CrossRef] [Green Version]
- Szunerits, S.; Barras, A.; Khanal, M.; Pagneux, Q.; Boukherroub, R. Nanostructures for the Inhibition of Viral Infections. Molecules 2015, 20, 14051–14081. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Williams, G.R.; Touabi, L.; Mclean, G.R. An Investigation of Rhinovirus Infection on Cellular Uptake of Poly (Glycerol-Adipate) Nanoparticles. Int. J. Pharm. 2020, 589, 119826. [Google Scholar] [CrossRef]
- Hillaireau, H.; Le Doan, T.; Appel, M.; Couvreur, P. Hybrid Polymer Nanocapsules Enhance in Vitro Delivery of Azidothymidine-Triphosphate to Macrophages. J. Control. Release 2006, 116, 346–352. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Mantovani, G.; Irving, W.L.; Garnett, M.C. Synthesis of Nucleoside-Boronic Esters Hydrophobic pro-Drugs: A Possible Route to Improve Hydrophilic Nucleoside Drug Loading into Polymer Nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 46, 354–364. [Google Scholar] [CrossRef]
- Hashim, F.; El-Ridy, M.; Nasr, M.; Abdallah, Y. Preparation and Characterization of Niosomes Containing Ribavirin for Liver Targeting. Drug Deliv. 2010, 17, 282–287. [Google Scholar] [CrossRef]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in New Carboxylated Cyclodextrin-Based Nanosponges Improves the Agent’s Antiviral Efficacy. Int. J. Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Abo-zeid, Y.; Garnett, M.C. Polymer Nanoparticle as a Delivery System for Ribavirin: Do Nanoparticle Avoid Uptake by Red Blood Cells? J. Drug Deliv. Sci. Technol. 2020, 56, 101552. [Google Scholar] [CrossRef]
- Sobhy, Y.; Mady, M.; Mina, S.; Abo-zeid, Y. Phytochemical and Pharmacological Values of Two Major Constituents of Asparagus Species and Their Nano Formulations: A Review. J. Adv. Pharm. Res. 2022, 6, 94–106. [Google Scholar] [CrossRef]
- Burgess, K.; Li, H.; Abo-Zeid, Y.; Fatimah; Williams, G.R. The Effect of Molecular Properties on Active Ingredient Release from Electrospun Eudragit Fibers. Pharmaceutics 2018, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, Z.; Abo-zeid, Y.; Bear, J.C.; Davies, G.; Lei, X.; Williams, G.R. SiO2-Coated Layered Gadolinium Hydroxides for Simultaneous Drug Delivery and Magnetic Resonance Imaging. J. Solid State Chem. 2020, 286, 121291. [Google Scholar] [CrossRef]
- Rangayasami, A.; Kannan, K.; Murugesan, S.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Influence of Nanotechnology to Combat against COVID-19 for Global Health Emergency: A Review. Sensors Int. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.; McLean, G.R.; Hamdy, N.M. A Molecular Docking Study Repurposes FDA Approved Iron Oxide Nanoparticles to Treat and Control COVID-19 Infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Urbanowicz, R.A.; Thomson, B.J.; William, L.; Tarr, A.W.; Garnett, M.C. Enhanced Nanoparticle Uptake into Virus Infected Cells: Could Nanoparticles Be Useful in Antiviral Therapy? Int. J. Pharm. 2018, 547, 572–581. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Sai Krishna, M.; Nalluru, S.; Sampath, S.K. Nanotechnology: An Emerging Approach to Combat COVID-19. Emergent Mater. 2021, 4, 119–130. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Amer, A.; El-Houssieny, B.; Bakkar, M.; Sakran, W. Overview on Bacterial Resistance and Nanoparticles to Overcome Bacterial Resistance. J. Adv. Pharm. Res. 2021, 5, 312–326. [Google Scholar] [CrossRef]
- Lotfabad, T.B.; Shahcheraghi, F.; Shooraj, F. Assessment of Antibacterial Capability of Rhamnolipids Produced by Two Indigenous Pseudomonas aeruginosa Strains. Jundishapur J. Microbiol. 2013, 6, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Carrazco-Palafox, J.; Rivera-Chavira, B.E.; Adame-Gallegos, J.R.; Rodríguez-Valdez, L.M.; Orrantia-Borunda, E.; Nevárez-Moorillón, G.V. Rhamnolipids from Pseudomonas aeruginosa Rn19a Modifies the Biofilm Formation over a Borosilicate Surface by Clinical Isolates. Coatings 2021, 11, 136. [Google Scholar] [CrossRef]
- Sana, S.; Datta, S.; Biswas, D.; Sengupta, D. Assessment of Synergistic Antibacterial Activity of Combined Biosurfactants Revealed by Bacterial Cell Envelop Damage. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 579–585. [Google Scholar] [CrossRef]
- Remichkova, M.; Galabova, D.; Roeva, I.; Karpenko, E.; Shulga, A.; Galabov, A.S. Anti-Herpesvirus Activities of Pseudomonas sp. S-17 Rhamnolipid and Its Complex with Alginate. Z. Nat. C 2008, 63, 75–81. [Google Scholar] [CrossRef]
- Buonocore, C.; Tedesco, P.; Vitale, G.A.; Esposito, F.P.; Giugliano, R.; Monti, M.C.; D’Auria, M.V.; de Pascale, D. Characterization of a New Mixture of Mono-Rhamnolipids Produced by Pseudomonas gessardii Isolated from Edmonson Point (Antarctica). Mar. Drugs 2020, 18, 269. [Google Scholar] [CrossRef]
- Akbari, B.; Tavandashti, M.P.; Zandrahimi, M. Particle Size Characterization of Nanoparticles—A Practicalapproach. Iran. J. Mater. Sci. Eng. 2011, 8, 48–56. [Google Scholar]
- Putri, D.C.A.; Dwiastuti, R.; Marchaban, M.; Nugroho, A.K. Optimization of Mixing Temperature and Sonication Duration in Liposomes Preparation. J. Pharm. Sci. Community 2017, 14, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Landucci, E.; Bilia, A.R.; Bergonzi, M.C. Formulation of Nanomicelles to Improve the Solubility and the Oral Absorption of Silymarin. Molecules 2019, 24, 1688. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Yang, X.P.; Ma, L.Z. Analysis of Biosurfactants from Industrially Viable Pseudomonas Strain Isolated from Crude Oil Suggests How Rhamnolipids Congeners Affect Emulsification Property and Antimicrobial Activity. Front. Microbiol. 2014, 5, 696. [Google Scholar] [CrossRef]
- Surini, S.; Amirtha, N.I.; Lestari, D.C. Formulation and Effectiveness of a Hand Sanitizer Gel Produced Using Salam Bark Extract. Int. J. Appl. Pharm. 2018, 10, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.M.; Yosipovitch, G. Skin PH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Aodah, A.H.; Bakr, A.A.; Booq, R.Y.; Rahman, M.J.; Alzahrani, D.A.; Alsulami, K.A.; Alshaya, H.A.; Alsuabeyl, M.S.; Alyamani, E.J.; Tawfik, E.A. Preparation and Evaluation of Benzalkonium Chloride Hand Sanitizer as a Potential Alternative for Alcohol-Based Hand Gels. Saudi Pharm. J. 2021, 29, 807–814. [Google Scholar] [CrossRef]
- Booq, R.Y.; Alshehri, A.A.; Almughem, F.A.; Zaidan, N.M.; Aburayan, W.S.; Bakr, A.A.; Kabli, S.H.; Alshaya, H.A.; Alsuabeyl, M.S.; Alyamani, E.J.; et al. Formulation and Evaluation of Alcohol-Free Hand Sanitizer Gels to Prevent the Spread of Infections during Pandemics. Int. J. Environ. Res. Public Health 2021, 18, 6252. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal Ph of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Choudhary, S.; Malik, Y.S.; Tomar, S. Identification of SARS-CoV-2 Cell Entry Inhibitors by Drug Repurposing Using in Silico Structure-Based Virtual Screening Approach. Front. Immunol. 2020, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Chrzanowski; Pijanowska, A.; Olszanowski, A. Yeast and Bacteria Cell Hydrophobicity and Hydrocarbon Biodegradation in the Presence of Natural Surfactants: Rhamnolipides and Saponins. Bioresour. Technol. 2008, 99, 4285–4291. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.M.; Shi, J.G.; Yuan, X.Z.; Liu, J.; Zhang, Z.B.; Huang, G.H.; Li, J.B.; Xi, B.D.; Liu, H.L. Effects of Tween 80 and Rhamnolipid on the Extracellular Enzymes of Penicillium simplicissimum Isolated from Compost. Enzyme Microb. Technol. 2006, 39, 1451–1456. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, Z.; Zhong, H.; Li, J.; Yuan, X.; Fu, H.; Ding, Y.; Wang, J.; Zhou, M. Effect of Monorhamnolipid on the Degradation of N-Hexadecane by Candida Tropicalis and the Association with Cell Surface Properties. Appl. Microbiol. Biotechnol. 2011, 90, 1155–1161. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zhong, H.; Zeng, G.; Liu, G.; Yu, M.; Liu, Y.; Yang, X.; Li, Z.; Fang, Z.; et al. Effects of Rhamnolipids on Microorganism Characteristics and Applications in Composting: A Review. Microbiol. Res. 2017, 200, 33–44. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B. Interactions of Surfactants and Fatty Acids with Lipids. Curr. Opin. Colloid Interface Sci. 2001, 6, 277–286. [Google Scholar] [CrossRef]
- Chaudhary, N.K.; Chaudhary, N.; Dahal, M.; Guragain, B.; Rai, S.; Chaudhary, R.; Sachin, K.; Lamichhane-Khadka, R.; Bhattarai, A. Fighting the SARS CoV-2 (COVID-19) Pandemic with Soap. Preprints 2020, 2020050060. [Google Scholar] [CrossRef]
- Bharali, P.; Das, S.; Ray, A.; Pradeep Singh, S.; Bora, U.; Kumar Konwar, B.; Singh, C.B.; Sahoo, D. Biocompatibility Natural Effect of Rhamnolipids in Bioremediation Process on Different Biological Systems at the Site of Contamination. Bioremediat. J. 2018, 22, 91–102. [Google Scholar] [CrossRef]
- Monteiro, S.A.; Sassaki, G.L.; de Souza, L.M.; Meira, J.A.; de Araújo, J.M.; Mitchell, D.A.; Ramos, L.P.; Krieger, N. Molecular and Structural Characterization of the Biosurfactant Produced by Pseudomonas aeruginosa DAUPE 614. Chem. Phys. Lipids 2007, 147, 1–13. [Google Scholar] [CrossRef]
- Rahman, P.K.S.M.; Pasirayi, G.; Auger, V.; Ali, Z.; Rahman, K.S.M. Production of Rhamnolipid Biosurfactants by Pseudomonas aeruginosa DS10-129 in a Microfluidic Bioreactor. Biotechnol. Appl. Biochem. 2010, 55, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Abraham, M.H.; Hassanisadi, M.; Jalali-Heravi, M.; Ghafourian, T.; Cain, W.S.; Cometto-Muñiz, J.E. Draize Rabbit Eye Test Compatibility with Eye Irritation Thresholds in Humans: A Quantitative Structure-Activity Relationship Analysis. Toxicol. Sci. 2003, 76, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Ameeduzzafar; Syed Sarim, I.; Abbas Bukhari, S.N.; Ahmad, J.; Ali, A. Formulation and Optimization of Levofloxacin Loaded Chitosan Nanoparticle for Ocular Delivery: In-Vitro Characterization, Ocular Tolerance and Antibacterial Activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef]
- Guideline, O.; The, F.O.R.; Of, T. OECD 404 Guidelines for Testing of Chemicals—Acute Dermal Irritation/Corrotion; Organization for Economic Cooperation and Development: Paris, France, 2002; pp. 1–13. [Google Scholar]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Hirasawa, Y.; Ohtsu, S.; Matsui, Y.; Nagase, T.; Shimizu, M.; Masaaki, O.; Kyuki, K.; Takahashi, T.; Takahashi, K. Assessing Effects of a Product Containing Crude Drugs Including Scutellaria Root, Phellodendron Bark, Coptis Rhizome and Product Containing Crude Drugs Including Lithospermum Root, Japanese Angelica Root, Sesame Oil in Atopic Dermatitis NC/Nga Mice. Pharmacometrics 2000, 59, 123–134. [Google Scholar]










| Control Site | Treated Site | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythema and Eschar | Edema | Erythema and Eschar | Edema | ||||||||||||||
| Intact | Abraded | Intact | Abraded | Intact | Abraded | Intact | Abraded | ||||||||||
| Tested sample/tested time (h) | Animal Number | 24 | 72 | 24 | 72 | 24 | 72 | 24 | 72 | 24 | 72 | 24 | 72 | 24 | 72 | 24 | 72 |
| Rhamnolipids nano-micelles (0.625 µg/mL) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PBS (10 mM, pH 7.4) | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tested Solution | Tissues Examined in the Eye | Number of Rabbits | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| RT. Untreated | LT. Treated | RT. Untreated | LT. Treated | RT. Untreated | LT. Treated | ||
| Rhamnolipids nano-micelles (0.625 mg/mL) | Cornea | 0 | 0 | 0 | 0 | 0 | 0 |
| Iris | 0 | 0 | 0 | 0 | 0 | 0 | |
| Conjunctiva | 0 | 0 | 0 | 0 | 0 | 0 | |
| PBS (10 mM, pH 7.4) | Cornea | 0 | 0 | 0 | 0 | 0 | 0 |
| Iris | 0 | 0 | 0 | 0 | 0 | 0 | |
| Conjunctiva | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hours/ Number of Animals | Positive Control Erythema/Edema | Rhamnolipids Nano-Micelles (0.625 mg/mL) Erythema/Edema | Negative Control Erythema/Edema | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | |
| 1 | 0/0 | 2/1 | 2/2 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| 2 | 0/0 | 2/1 | 2/2 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| 3 | 0/0 | 2/1 | 2/3 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| 4 | 0/0 | 3/1 | 2/3 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| 5 | 0/0 | 3/1 | 3/3 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| 6 | 0/0 | 0/0 | |||||||
| 7 | 0/0 | 0/0 | |||||||
| 8 | 0/0 | 0/0 | |||||||
| 9 | 0/0 | 0/0 | |||||||
| 10 | 0/0 | 0/0 | |||||||
| Animal Group | AST (U/L) | ALT (U/L) |
|---|---|---|
| Untreated animal (Negative Control) | 30.00 ± 1.53 | 27.33 ± 1.20 |
| PBS | 32.70 ± 3.20 | 28.20 ± 2.80 |
| Rhamnolipids nano-micelles (0.625 mg/mL) | 35.00 ± 4.35 | 28.67 ± 3.71 |
| Animal Groups | PCV (%) | Hb (g/dL) | RBCs × 106/µL | MCV (fL) | MCHC (%) |
|---|---|---|---|---|---|
| Untreated animal (Control) | 38.53 ± 0.56 | 12.00 ± 0.64 | 8.84 ± 0.11 | 48.87±0.22 | 37.26 ± 0.46 |
| PBS | 37.90 ± 0.61 | 11.51 ± 0.32 | 7.80 ± 0.13 | 48.20 ± 0.19 | 37.3 ± 0.32 |
| Rhamnolipids nano-micelles (0.625 mg/mL) | 39.41± 0.26 | 11.34 ± 0.20 | 7.61±0.140 | 47.72 ± 0.26 | 37.28 ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.M.; Hill, H.J.; Elkhouly, G.E.; Bakkar, M.R.; Raya, N.R.; Stamataki, Z.; Abo-zeid, Y. Rhamnolipid Nano-Micelles Inhibit SARS-CoV-2 Infection and Have No Dermal or Eye Toxic Effects in Rabbits. Antibiotics 2022, 11, 1556. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11111556
Ali AM, Hill HJ, Elkhouly GE, Bakkar MR, Raya NR, Stamataki Z, Abo-zeid Y. Rhamnolipid Nano-Micelles Inhibit SARS-CoV-2 Infection and Have No Dermal or Eye Toxic Effects in Rabbits. Antibiotics. 2022; 11(11):1556. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11111556
Chicago/Turabian StyleAli, Alaa M., Harriet J. Hill, Gehad E. Elkhouly, Marwa Reda Bakkar, Nermeen R. Raya, Zania Stamataki, and Yasmin Abo-zeid. 2022. "Rhamnolipid Nano-Micelles Inhibit SARS-CoV-2 Infection and Have No Dermal or Eye Toxic Effects in Rabbits" Antibiotics 11, no. 11: 1556. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11111556