Quantifying Antibiotic Distribution in Solid and Liquid Fractions of Manure Using a Two-Step, Multi-Residue Antibiotic Extraction
Abstract
:1. Introduction
2. Results and Discussions
2.1. Comparative Analysis of Buffer vs Solvent Extraction Efficacy
2.2. Initial Two-Step Extraction with Buffer Followed by Methanol
2.3. Two-Step Extraction on Solid and Aqueous Manure Samples Based on Total Solids
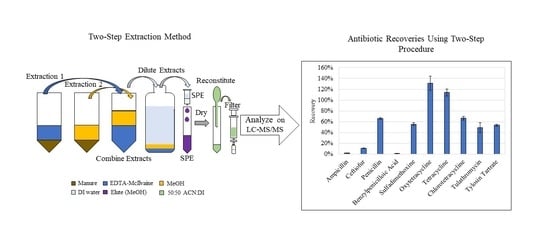
2.4. Optimizing the Two-Step Method to Combine Extracts for One Injection
2.5. Extraction Method Performance Using Method A
3. Materials and Methods
3.1. Standards and Reagents
3.2. Collection of Dairy Manure Samples
3.3. Sample Preparation
3.4. Sample Cleanup
3.5. UPLC-MS/MS Conditions
3.6. Method Trials
3.6.1. Comparative Analysis of Buffer vs. Solvent Extraction Efficacy
3.6.2. Initial Two-Step Extraction with Buffer Followed by Methanol
3.6.3. Two-Step Extraction on Solid and Aqueous Manure Samples Based on Total Solids
3.6.4. Optimizing the Two-Step Method to Combine Extracts for One Injection
3.7. Evaluation of Performance of Method Trials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spielmeyer, A. Occurrence and fate of antibiotics in manure during manure treatments: A short review. Sustain. Chem. Pharm. 2018, 9, 76–86. [Google Scholar] [CrossRef]
- CVM. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; USFDA: Rockville, MD, USA; Washington, DC, USA, 2019; pp. 1–49.
- 86 FR 31317; Recommendations for Sponsors of Medically Important Antimicrobial Drugs Approved for Use in Animals to Voluntarily Bring under Veterinary Oversight All Products That Continue to Be Available Over-the-Counter. CVM: Silver Spring, MD, USA, 2021.
- Wallace, J.S.; Aga, D.S. Enhancing Extraction and Detection of Veterinary Antibiotics in Solid and Liquid Fractions of Manure. J. Environ. Qual. 2016, 45, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.L.; Garcia, H.; Rico, C.; Tejero, I. Characterisation of solid and liquid fractions of dairy manure with regard to their component distribution and methane production. Bioresour. Technol. 2007, 98, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.E.; Morra, M.J.; Parikh, S.J. Pressurized liquid extraction of six tetracyclines from agricultural soils. J. Environ. Sci. Health B 2019, 54, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Tian, H.; Wei, Q.; Liu, B.; Bao, G.; Liao, M.; Peng, J.; Huang, X.; Wang, L. High through-put determination of 28 veterinary antibiotic residues in swine wastewater by one-step dispersive solid phase extraction sample cleanup coupled with ultra-performance liquid chromatography-tandem mass spectrometry. Chemosphere 2019, 230, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.J.M.; van de Schans, M.G.M.; de Boer, D.; Bongers, I.E.A.; Schmitt, H.; Hoeksma, P.; Berendsen, B.J.A. A new extraction procedure to abate the burden of non-extractable antibiotic residues in manure. Chemosphere 2019, 224, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, B.J.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef]
- Jacobsen, A.M.; Halling-Sorensen, B. Multi-component analysis of tetracyclines, sulfonamides and tylosin in swine manure by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2006, 384, 1164–1174. [Google Scholar] [CrossRef]
- Karaca, S.; Kabil, E.; Akmehmet Balcıoğlu, I. Quantification of multi-class antibiotics by UHPLC–MS/MS analysis combined with salt-assisted acetonitrile extraction: Comparative evaluation of dairy and poultry manure. Int. J. Environ. Anal. Chem. 2018, 98, 1186–1206. [Google Scholar] [CrossRef]
- Pan, X.; Qiang, Z.; Ben, W.; Chen, M. Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in Shandong Province, China. Chemosphere 2011, 84, 695–700. [Google Scholar] [CrossRef]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Simultaneous determination of veterinary antibiotics and hormone in broiler manure, soil and manure compost by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Occurrence of veterinary antibiotics and progesterone in broiler manure and agricultural soil in Malaysia. Sci. Total Environ. 2014, 488–489, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Van den Meersche, T.; Van Pamel, E.; Van Poucke, C.; Herman, L.; Heyndrickx, M.; Rasschaert, G.; Daeseleire, E. Development, validation and application of an ultra high performance liquid chromatographic-tandem mass spectrometric method for the simultaneous detection and quantification of five different classes of veterinary antibiotics in swine manure. J. Chromatogr. A 2016, 1429, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, C.; Zhang, W.; Liu, Y.; Li, Z.; Hu, H.; Xue, J.; Davis, M. A simple and economic method for simultaneous determination of 11 antibiotics in manure by solid-phase extraction and high-performance liquid chromatography. J. Soils Sediments 2016, 16, 2242–2251. [Google Scholar] [CrossRef]
- Kim, H.; Hong, Y.; Park, J.E.; Sharma, V.K.; Cho, S.I. Sulfonamides and tetracyclines in livestock wastewater. Chemosphere 2013, 91, 888–894. [Google Scholar] [CrossRef]
- Hu, F.Y.; He, L.M.; Yang, J.W.; Bian, K.; Wang, Z.N.; Yang, H.C.; Liu, Y.H. Determination of 26 veterinary antibiotics residues in water matrices by lyophilization in combination with LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 949–950, 79–86. [Google Scholar] [CrossRef]
- Kemper, N.; Färber, H.; Skutlarek, D.; Krieter, J. Analysis of antibiotic residues in liquid manure and leachate of dairy farms in Northern Germany. Agric. Water Manag. 2008, 95, 1288–1292. [Google Scholar] [CrossRef]
- Wang, R.; Feng, F.; Chai, Y.; Meng, X.; Sui, Q.; Chen, M.; Wei, Y.; Qi, K. Screening and quantitation of residual antibiotics in two different swine wastewater treatment systems during warm and cold seasons. Sci. Total Environ. 2019, 660, 1542–1554. [Google Scholar] [CrossRef]
- Zhou, L.J.; Ying, G.G.; Liu, S.; Zhao, J.L.; Chen, F.; Zhang, R.Q.; Peng, F.Q.; Zhang, Q.Q. Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2012, 1244, 123–138. [Google Scholar] [CrossRef]
- Wallace, J.S.; Garner, E.; Pruden, A.; Aga, D.S. Occurrence and transformation of veterinary antibiotics and antibiotic resistance genes in dairy manure treated by advanced anaerobic digestion and conventional treatment methods. Environ. Pollut. 2018, 236, 764–772. [Google Scholar] [CrossRef]
- McKinney, C.W.; Loftin, K.A.; Meyer, M.T.; Davis, J.G.; Pruden, A. tet and sul antibiotic resistance genes in livestock lagoons of various operation type, configuration, and antibiotic occurrence. Environ. Sci. Technol. 2010, 44, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Xian-Gang, H.; Yi, L.; Qi-Xing, Z.; Lin, X. Determination of Thirteen Antibiotics Residues in Manure by Solid Phase Extraction and High Performance Liquid Chromatography. Chin. J. Anal. Chem. 2008, 36, 1162–1166. [Google Scholar]
- Gros, M.; Mas-Pla, J.; Boy-Roura, M.; Geli, I.; Domingo, F.; Petrovic, M. Veterinary pharmaceuticals and antibiotics in manure and slurry and their fate in amended agricultural soils: Findings from an experimental field site (Baix Emporda, NE Catalonia). Sci. Total Environ. 2019, 654, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.J.; Wallace, J.S.; Aga, D.S. Method development for the analysis of ionophore antimicrobials in dairy manure to assess removal within a membrane-based treatment system. Chemosphere 2018, 197, 271–279. [Google Scholar] [CrossRef]
- Guo, X.Y.; Hao, L.J.; Qiu, P.Z.; Chen, R.; Xu, J.; Kong, X.J.; Shan, Z.J.; Wang, N. Pollution characteristics of 23 veterinary antibiotics in livestock manure and manure-amended soils in Jiangsu province, China. J. Environ. Sci. Health B 2016, 51, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cui, Y.; Tao, Y.; Huang, L.; Peng, D.; Xie, S.; Wang, X.; Liu, Z.; Chen, D.; Yuan, Z. Multiclass method for the quantification of 92 veterinary antimicrobial drugs in livestock excreta, wastewater, and surface water by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 4086–4095. [Google Scholar] [CrossRef] [PubMed]
- US EPA. CompTox Chemicals Dashboard. Available online: https://comptox.epa.gov/dashboard (accessed on 21 July 2021).
- Zrncic, M.; Babic, S.; Mutavdzic Pavlovic, D. Determination of thermodynamic pKa values of pharmaceuticals from five different groups using capillary electrophoresis. J. Sep. Sci. 2015, 38, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Schmidt, T.C. Determination of acid dissociation constants (pKa) of cephalosporin antibiotics: Computational and experimental approaches. Chemosphere 2017, 169, 524–533. [Google Scholar] [CrossRef]
- Geiser, L.; Henchoz, Y.; Galland, A.; Carrupt, P.A.; Veuthey, J.L. Determination of pKa values by capillary zone electrophoresis with a dynamic coating procedure. J. Sep. Sci. 2005, 28, 2374–2380. [Google Scholar] [CrossRef]
- Villarino, N.; Brown, S.A.; Martín-Jiménez, T. The role of the macrolide tulathromycin in veterinary medicine. Vet. J. 2013, 198, 352–357. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Kazemi-Vaysari, A.; Ardjmand, M.; Baradar-Khoshfetrat, A. The combined effects of pH and temperature on penicillin G decomposition and its stability modeling. Process Biochem. 1999, 35, 205–211. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.Y.; Liu, Y.S.; Zhao, J.L.; Liu, W.R.; Zhang, J.N.; Chen, J.; He, L.K.; Zhang, Q.Q.; Ying, G.G. Fate of veterinary antibiotics during animal manure composting. Sci. Total Environ. 2019, 650, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, B.J.A.; Lahr, J.; Nibbeling, C.; Jansen, L.J.M.; Bongers, I.E.A.; Wipfler, E.L.; van de Schans, M.G.M. The persistence of a broad range of antibiotics during calve, pig and broiler manure storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.P.; Gooch, C.A.; Lansing, S.; Schueler, J.; Hurst, J.J.; Sassoubre, L.; Crossette, E.M.; Aga, D.S. Invited review: Fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J. Dairy Sci. 2020, 103, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.P.; Hurst, J.J.; Gooch, C.A.; Stappenbeck, A.; Sassoubre, L.; Aga, D.S. On-farm screw-press/rotary drum treatment of dairy manure associated antibiotic residues and resistance. J. Environ. Qual. 2021, 50, 134–143. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Martinez-Carballo, E.; Gonzalez-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef]
- Arikan, O.A.; Sikora, L.J.; Mulbry, W.; Khan, S.U.; Rice, C.; Foster, G.D. The fate and effect of oxytetracycline during the anaerobic digestion of manure from therapeutically treated calves. Process Biochem. 2006, 41, 1637–1643. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Stolker, L.A.M.; Nielen, M.W.F.; Nielen, M.W.F. Selectivity in the sample preparation for the analysis of drug residues in products of animal origin using LC-MS. TrAC Trends Anal. Chem. 2013, 43, 229–239. [Google Scholar] [CrossRef]
- Araujo, P. Key aspects of analytical method validation and linearity evaluation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2224–2234. [Google Scholar] [CrossRef]




| Antibiotics | Acronym | Internal Standard | Molecular Weight (g/mol) | Precursor Ion (m/z) | Product Ion (m/z) | RT (min) | Log Kow b | pKa |
|---|---|---|---|---|---|---|---|---|
| β-Lactams | ||||||||
| Ampicillin | AMP | NA | 349.41 | 350.1 | 106.1 a, 114.0 | 1.88 | 1.35 | 3.07, 7.12 c |
| Ceftiofur | CEF | NA | 523.56 | 524.0 | 241.0 a, 125.2 | 2.03 | 1.22 | 2.64, 3.44, 10.7 d |
| Penicillin-G | PEN | NA | 334.39 | 335.1 | 217.1 a, 160.0 | 2.13 | 1.83 | 2.97, 4.75 c |
| Benzylpenicilloic Acid | BEN | NA | 352.4 | 353.1 | 160.0 a, 128.0 | 2.12 | ND | ND |
| Sulfonamides | ||||||||
| Sulfadimethoxine | SUL | SUL-d6 | 310.33 | 311.1 | 156.1 a, 92.1 | 2.11 | 1.63 | 1.62, 6.13 e |
| Tetracyclines | ||||||||
| Oxytetracycline | OXY | DEM | 460.44 | 461.2 | 201.1 a, 98.1 | 1.89 | −0.9 | 3.71, 8.08, 10.15 b |
| Tetracycline | TET | DEM | 444.3 | 445.0 | 410.2 a, 154.1 | 1.89 | −1.37 | 3.56, 7.09, 9.28 c |
| Chlorotetracycline | CHL | DEM | 478.88 | 479.1 | 154.1 a, 98 | 1.94 | −0.62 | 3.49, 7.14, 9.28 c |
| Macrolides | ||||||||
| Tulathromycin-A | TUL | NAL | 806.1 | 403.9 | 72.1 a, 116.1 | 1.86 | 3.69 | 8.6–9.6 e |
| Tylosin Tartrate | TYL | ROX | 916.112 | 916.5 | 174.1 a, 101.0 | 1.97 | 1.95 | 7.71c |
| Internal Standards | ||||||||
| Sulfadimethoxine-d6 | SUL-d6 | NA | 316.37 | 317.95 | 108.0 a | 2.11 | ||
| Demeclocycline | DEM | NA | 464.86 | 465.1 | 154.1 a | 1.92 | ||
| Nalidixic acid-d5 | NAL | NA | 237.27 | 238.24 | 104.2 a | 2.23 | ||
| Roxithromycin | ROX | NA | 837.06 | 837.54 | 158.1 a | 2.07 | ||
| Manure | Total Solids (gsolids/gwet manure) | Mass Extracted (gwet) |
|---|---|---|
| Blank Dairy Manure (BDM) | 0.134 ± 0.005 | 2 |
| Unprocessed Pit Manure (UPM) | 0.074 ± 0.004 | 3.61 |
| Separated Liquid (SL) | 0.064 ± 0.0002 | 4.20 |
| Separated Solids (SS) | 0.347 ± 0.010 | 0.770 |
| Bedding Recovery Unit (BRU) | 0.370 ± 0.120 | 0.722 |
| Antibiotic | Method A | Method B | p-Value |
|---|---|---|---|
| Recoveries (%) ± SD | |||
| Oxytetracycline | 131 ± 13 | 45 ± 4 | 0.0004 |
| Tetracycline | 114 ± 7 | 68 ± 2 | 0.0004 |
| Chlorotetracycline | 67 ± 3 | 54 ± 1 | 0.0021 |
| Penicillin-G | 66 ± 1 | 58 ± 1 | 0.0006 |
| Sulfadimethoxine | 56 ± 3 | 31 ± 2 | 0.0003 |
| Tylosin | 53 ± 2 | 47 ± 6 | 0.18 |
| Tulathromycin-A | 49 ± 9 | 43 ± 2 | 0.32 |
| Ceftiofur | 11 ± 0.3 | 6.4 ± 0.5 | 0.0002 |
| Ampicillin | 2.3 ± 0.1 | 7.3 ± 0.4 | 0.00003 |
| Benzylpenicilloic Acid | 1.3 ± 0.04 | 5.6 ± 0.4 | 0.0001 |
| Antibiotics | Recovery (%RSD), n = 3 | Matrix Effect | Linearity Fit (R2) | LOD a (µg/kg) | LOQ b (µg/kg) |
|---|---|---|---|---|---|
| AMP | 2% (4%) | 88% | 0.995 | 3.58 | 10.8 |
| CEF | 11% (21%) | 88% | 0.996 | 0.893 | 2.71 |
| PEN | 57% (22%) | 79% | 0.986 | 2.53 | 7.68 |
| BEN | 0.5% (31%) | 85% | 0.988 | 4.83 | 14.6 |
| SUL | 56% (8%) | 68% | 0.999 | 0.606 | 1.84 |
| OXY | 131% (17%) | 88% | 0.999 | 8.05 | 24.4 |
| TET | 114% (10%) | 88% | 0.999 | 2.02 | 6.11 |
| CHL | 66.2% (6%) | 89% | 0.999 | 7 | 21.2 |
| TUL | 47% (25%) | 57% | 0.989 | 3.18 | 9.64 |
| TYL | 55% (3%) | 86% | 0.996 | 0.229 | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poindexter, C.; Yarberry, A.; Rice, C.; Lansing, S. Quantifying Antibiotic Distribution in Solid and Liquid Fractions of Manure Using a Two-Step, Multi-Residue Antibiotic Extraction. Antibiotics 2022, 11, 1735. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11121735
Poindexter C, Yarberry A, Rice C, Lansing S. Quantifying Antibiotic Distribution in Solid and Liquid Fractions of Manure Using a Two-Step, Multi-Residue Antibiotic Extraction. Antibiotics. 2022; 11(12):1735. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11121735
Chicago/Turabian StylePoindexter, Carlton, Andrea Yarberry, Clifford Rice, and Stephanie Lansing. 2022. "Quantifying Antibiotic Distribution in Solid and Liquid Fractions of Manure Using a Two-Step, Multi-Residue Antibiotic Extraction" Antibiotics 11, no. 12: 1735. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11121735