Waterborne Polyurea Coatings Filled with Sulfonated Graphene Improved Anti-Corrosion Performance
Abstract
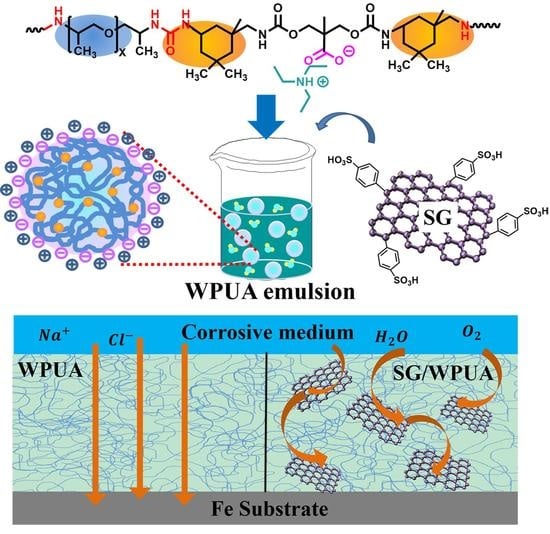
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Preparation of SG
2.3. Preparation of Waterborne Polyurea
2.4. Preparation of Composite Coatings
2.5. Characterization
3. Results and Discussion
3.1. Characterization of SG and WPUA
3.2. Dispersion Stability of GO and SG in WPUA
3.3. Water Resistance of Composite Films
3.4. Corrosion Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arunkumar, T.; Ramachandran, S. Surface coating and characterisation of polyurea for liquid storage. Int. J. Ambient Energy 2017, 38, 781–787. [Google Scholar] [CrossRef]
- Wang, H.; Deng, X.; Wu, H.; Pi, A.; Li, J.; Huang, F. Investigating the dynamic mechanical behaviors of polyurea through experimentation and modeling. Def. Technol. 2019, 15, 875–884. [Google Scholar] [CrossRef]
- Iqbal, N.; Sharma, P.K.; Kumar, D.; Roy, P.K. Protective polyurea coatings for enhanced blast survivability of concrete. Constr. Build. Mater. 2018, 175, 682–690. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Zhao, T.; Cao, X.; Jiang, Y.; Xiao, D.; Fang, D. An experimental study on dynamic response of polyurea coated metal plates under intense underwater impulsive loading. Int. J. Impact Eng. 2019, 133, 103361. [Google Scholar] [CrossRef]
- Iqbal, N.; Kumar, D.; Roy, P.K. Understanding the role of isocyanate dilution toward spraying of polyurea. J. Appl. Polym. Sci. 2018, 135, 1–8. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Rinaldi, R.G.; Boyce, M.C.; Weigand, S.J.; Londono, D.J.; Guise, M.W. Microstructure evolution during tensile loading histories of a polyurea. J. Polym. Sci. Polym. Phys. 2011, 49, 1660–1671. [Google Scholar] [CrossRef]
- Li, T.; Zheng, T.; Han, J.; Liu, Z.; Guo, Z.X.; Zhuang, Z.; Xu, J.; Guo, B.H. Effects of diisocyanate structure and disulfide chain extender on hard segmental packing and self-healing property of polyurea elastomers. Polymers 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Lin, Y.; Li, G. An intermolecular quadruple hydrogen-bonding strategy to fabricate self-healing and highly deformable polyurethane hydrogels. J. Mater. Chem. 2014, 2, 6878–6885. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, Q.; Ma, C.; Zhang, G. Fouling release property of polydimethylsiloxane-based polyurea with improved adhesion to substrate. Ind. Eng. Chem. Res. 2016, 55, 6671–6676. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Dudita, M.; Bogatu, C.; Enesca, A.; Duta, A. The influence of the additives composition and concentration on the properties of SnOx thin films used in photocatalysis. Mater. Lett. 2011, 65, 2185–2189. [Google Scholar] [CrossRef]
- He, P.; Sun, J.; Tian, S.; Yang, S.; Ding, S.; Ding, G.; Xie, X.; Jiang, M. Processable aqueous dispersions of graphene stabilized by graphene quantum dots. Chem. Mater. 2015, 27, 218–226. [Google Scholar] [CrossRef]
- Gu, L.; Liu, S.; Zhao, H.; Yu, H. Facile preparation of water-dispersible graphene sheets stabilized by carboxylated oligoanilines and their anticorrosion coatings. ACS Appl. Mater. Interfaces 2015, 7, 17641–17648. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.B.; Luo, Z.; Zhang, X.; Dhamodharan, D.; Divakaran, N.; Mubarak, S.; Wu, L.; Xu, Y. Waterborne polyurethane/graphene oxide-silica nanocomposites with improved mechanical and thermal properties for leather coatings using screen printing. Polymer 2019, 170, 43–53. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Fei, G.; Wang, C.; Shen, Y.; Zhu, K.; Sun, L.; Rang, N.; Guo, D.; Wallace, G.G. Functionalizing graphene with titanate coupling agents as reinforcement for one-component waterborne poly(urethane-acrylate) anticorrosion coatings. Chem. Eng. J. 2019, 359, 331–343. [Google Scholar] [CrossRef]
- Appel, A.K.; Thomann, R.; Mülhaupt, R. Hydroxyalkylation and polyether polyol grafting of graphene tailored for graphene/polyurethane nanocomposites. Macromol. Rapid Commun. 2013, 34, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzadeh, B.; Bahlakeh, G.; Mohamadzadeh Moghadam, M.H.; Miraftab, R. Impact of size-controlled p-phenylenediamine (PPDA)-functionalized graphene oxide nanosheets on the GO-PPDA/Epoxy anti-corrosion, interfacial interactions and mechanical properties enhancement: Experimental and quantum mechanics investigations. Chem. Eng. J. 2018, 335, 737–755. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Wang, S.; Jiang, C.; Xie, Y.; Yang, M.; Shi, H. A novel and feasible approach for polymer amine modified graphene oxide to improve water resistance, thermal, and mechanical ability of waterborne polyurethane. Appl. Surf. Sci. 2019, 491, 301–312. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural defects in graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sheng, X.; Tian, Y.; Zhang, L.; Chen, Y.; Zhang, X. Two-dimensional nanomaterials for anticorrosive polymeric coatings: A review. Ind. Eng. Chem. Res. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Qiu, H.; Dai, Y.; Zheng, Q.; Zheng, G.-P.; Yang, J. Microwave-assisted simultaneous reduction and titanate treatment of graphene oxide. J. Mater. Chem. 2013, 1, 11451–11456. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Cuo, T.; Chen, Y. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Cui, J.; Qiu, H.; Yang, G.; Zheng, S.; Yang, J. Dispersion and parallel assembly of sulfonated graphene in waterborne epoxy anticorrosion coatings. J. Mater. Chem. 2019, 7, 17937–17946. [Google Scholar] [CrossRef]
- Liu, H.W.; Hu, S.H.; Chen, Y.W.; Chen, S.Y. Characterization and drug release behavior of highly responsive chip-like electrically modulated reduced graphene oxide-poly(vinyl alcohol) membranes. J. Mater. Chem. 2012, 22, 17311–17320. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Wang, X.; Shi, G. A pH-sensitive graphene oxide composite hydrogel. Chem. Commun. 2010, 46, 2376–2378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Hong, M.; Yang, Y.; Hu, N.; Zhou, Z.; Zhang, L.; Zhang, Y. Engineering sulfonated polyaniline molecules on reduced graphene oxide nanosheets for high-performance corrosion protective coatings. Appl. Surf. Sci. 2019, 484, 663–675. [Google Scholar] [CrossRef]
- Sun, W.; Wu, T.; Wang, L.; Yang, Z.; Zhu, T.; Dong, C.; Liu, G. The role of graphene loading on the corrosion-promotion activity of graphene/epoxy nanocomposite coatings. Compos. Eng. 2019, 173, 106916. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, S.; Chen, K.; Wang, J.; Wang, G.; Sun, K. Enhanced corrosion resistance of waterborne polyurethane containing sulfonated graphene/zinc phosphate composites. Prog. Org. Coat. 2019, 132, 409–416. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Concernng the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Jeyaprabha, C.; Muralidharan, S.; Venkatachari, G. Inhibition of iron corrosion in 0.5M sulphuric acid by metal cations. Appl. Surf. Sci. 2006, 252, 8107–8112. [Google Scholar] [CrossRef]










| Time (days) | Z-Ave (nm) | PDI | Peak (nm) |
|---|---|---|---|
| 7 | 177.7 | 0.065 | 191.7 |
| 14 | 178.3 | 0.089 | 194.3 |
| 28 | 177.1 | 0.109 | 195.5 |
| Samples | Ecorr (V) | icorr (μA/cm2) | vcorr (g/h) | η (%) |
|---|---|---|---|---|
| WPUA | −0.678 | 0.9772 | 0.8632 × 10−6 | — |
| 0.1-SG | −0.533 | 0.8340 | 0.3091 × 10−6 | 14.65 |
| 0.3-SG | −0.508 | 0.3192 | 0.2223 × 10−6 | 67.34 |
| 0.5-SG | −0.609 | 0.9018 | 0.5147 × 10−6 | 7.72 |
| 0.7-SG | −0.721 | 1.5834 | 2.534 × 10−6 | −62.03 |
| Time | Sample | CPEc | Rc (Ω·cm2) | CPEdl | Rct (Ω cm2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Y0 (Ω−1·cm−2·sn) | n | Cc (F·cm−2) | Y0 (Ω−1·cm−2·sn) | n | Cdl (F cm−2) | ||||
| 6 h | WPUA | 7.55 × 10−5 | 0.57 | 1.18 × 10−5 | 2.15 × 102 | — | — | — | — |
| 0.3-SG | 4.16 × 10−7 | 0.63 | 1.95 × 10−7 | 6.96 × 104 | — | — | — | — | |
| 24 h | WPUA | — | — | 1.20 × 10−5 | 2.70 | 9.80 × 10−5 | 0.66 | 9.04 × 10−5 | 7.74 × 103 |
| 0.3-SG | — | — | 1.39 × 10−6 | 15.15 | 1.76 × 10−5 | 0.86 | 1.25 × 10−5 | 8.61 × 104 | |
| 72 h | WPUA | — | — | 1.67 × 10−5 | 1.82 | 2.32 × 10−4 | 0.80 | 2.19 × 10−4 | 3.01 × 103 |
| 0.3-SG | — | — | 8.39 × 10−6 | 3.73 | 1.19 × 10−4 | 0.75 | 1.07 × 10−4 | 9.54 × 103 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, J.; Wen, S.; Li, S.; Chen, Y.; Wang, J.; Wang, Y.; Wang, C.; Yu, X.; Mao, Y. Waterborne Polyurea Coatings Filled with Sulfonated Graphene Improved Anti-Corrosion Performance. Coatings 2021, 11, 251. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11020251
Zhang J, Wang J, Wen S, Li S, Chen Y, Wang J, Wang Y, Wang C, Yu X, Mao Y. Waterborne Polyurea Coatings Filled with Sulfonated Graphene Improved Anti-Corrosion Performance. Coatings. 2021; 11(2):251. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11020251
Chicago/Turabian StyleZhang, Jijia, Jihu Wang, Shaoguo Wen, Siwei Li, Yabo Chen, Jing Wang, Yan Wang, Changrui Wang, Xiangyi Yu, and Yan Mao. 2021. "Waterborne Polyurea Coatings Filled with Sulfonated Graphene Improved Anti-Corrosion Performance" Coatings 11, no. 2: 251. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11020251