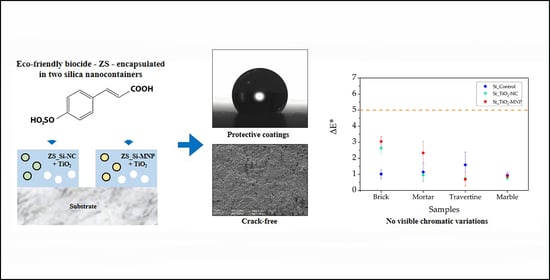
Assessment of Stone Protective Coatings with a Novel Eco-Friendly Encapsulated Biocide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Composition and Application of Coatings
2.3. Assessment of Coated Stone Performance and Compatibility
2.3.1. Static Contact Angle (SCA)
2.3.2. Colorimetric Measurements (CM)
2.3.3. Water Absorption through Capillarity (WAC)
2.3.4. Water Vapor Permeability (WVP)
2.3.5. Optical Surface Roughness (OSR)
2.3.6. Scanning Electron Microscopy (SEM) Combined to Energy Dispersive Spectroscopy (SEM-EDS)
2.3.7. Photocatalysis Testing (PT)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camuffo, D. Chapter 7—Atmospheric Water, Capillary Rise, and Stone Weathering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Saad, A.; Guédon, S.; Martineau, F. Microstructural weathering of sedimentary rocks by freeze-thaw cycles: Experimental study of state and transfer parameters. Comptes Rendus—Geosci. 2010, 342, 197–203. [Google Scholar] [CrossRef]
- Steiger, M.; Charola, E. Chapter 4—Weathering and deterioration. In Stone in Architecture; Siegesmund, R.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 227–316. [Google Scholar]
- Traversetti, L.; Bartoli, F.; Caneva, G. Wind-driven rain as a bioclimatic factor affecting the biological colonization at the archaeological site of Pompeii, Italy. Int. Biodeterior. Biodegrad. 2018, 134, 31–38. [Google Scholar] [CrossRef]
- Caneva, G.; Bartoli, F.; Ceschin, S.; Salvadori, O.; Futagami, Y.; Salvati, L. Exploring ecological relationships in the biodeterioration patterns of Angkor temples (Cambodia) along a forest canopy gradient. J. Cult. Herit. 2015, 16, 728–735. [Google Scholar] [CrossRef]
- Caneva, G.; Bartoli, F.; Savo, V.; Futagami, Y.; Strona, G. Combining statistical tools and ecological assessments in the study of biodeterioration patterns of stone temples in angkor (Cambodia). Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiano, P. Biodeterioration of stone monuments a worldwide issue. Open Conf. Proc. J. 2016, 7, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Casanova Municchia, A.; Bartoli, F.; Taniguchi, Y.; Giordani, P.; Caneva, G. Evaluation of the biodeterioration activity of lichens in the Cave Church of Üzümlü (Cappadocia, Turkey). Int. Biodeterior. Biodegrad. 2018, 127, 160–169. [Google Scholar] [CrossRef]
- Urzi, C.; De Leo, F.; Krakova, L.; Pangallo, D.; Bruno, L. Effects of biocide treatments on the biofilm community in Domitilla’s catacombs in Rome. Sci. Total Environ. 2016, 572, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Lee, H.J.; Kim, D.W.; Chung, Y.J. New biocide for eco-friendly biofilm removal on outdoor stone monuments. Int. Biodeterior. Biodegrad. 2018, 131, 19–28. [Google Scholar] [CrossRef]
- Toreno, G.; Isola, D.; Meloni, P.; Carcangiu, G.; Selbmann, L.; Onofri, S.; Caneva, G.; Zucconi, L. Biological colonization on stone monuments: A new low impact cleaning method. J. Cult. Herit. 2018, 30, 100–109. [Google Scholar] [CrossRef]
- Young, M.E.; Alakomi, H.L.; Fortune, I.; Gorbushina, A.A.; Krumbein, W.E.; Maxwell, I.; McCullagh, C.; Robertson, P.; Saarela, M.; Valero, J.; et al. Development of a biocidal treatment regime to inhibit biological growths on cultural heritage: BIODAM. Environ. Geol. 2008, 56, 631–641. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Pinna, D. Coping with Biological Growth on Stone Heritage Objects: Methods, Products, Applications, and Perspectives; Apple Academic Press: Palm Bay, FL, USA, 2017; ISBN 978-354-077-3-405. [Google Scholar]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Constr. Build. Mater. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; Ricca, M.; Macchia, A.; La Russa, M.F. Antifouling coatings for underwater archaeological stone materials. Prog. Org. Coat. 2017, 104, 64–71. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, B.; Galeotti, M. Monitoring the performance of innovative and traditional biocides mixed with consolidants and water-repellents for the prevention of biological growth on stone. Sci. Total Environ. 2012, 423, 132–141. [Google Scholar] [CrossRef]
- Geiger, T.; Delavy, P.; Hany, R.; Schleuniger, J. Encapsulated Zosteric Acid Embedded in Poly [3- hydroxyalkanoate] Coatings—Protection against Biofouling. Polym. Bull. 2004, 52, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Boopalan, M.; Sasikumar, A. Studies on Biocide Free and Biocide Loaded Zeolite Hybrid Polymer Coatings on Zinc Phosphated Mild Steel for the Protection of Ships Hulls from Biofouling and Corrosion. Silicon 2011, 3, 207–214. [Google Scholar] [CrossRef]
- Laabir, M.; Grignon-Dubois, M.; Masseret, E.; Rezzonico, B.; Soteras, G.; Rouquette, M.; Rieuvilleneuve, F.; Cecchi, P. Algicidal effects of Zostera marina L. and Zostera noltii Hornem. extracts on the neuro-toxic bloom-forming dinoflagellate Alexandrium catenella. Aquat. Bot. 2013, 111, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Villa, F.; Pitts, B.; Stewart, P.S.; Giussani, B.; Roncoroni, S.; Albanese, D.; Giordano, C.; Tunesi, M.; Cappitelli, F. Efficacy of Zosteric Acid Sodium Salt on the Yeast Biofilm Model Candida albicans. Microb. Ecol. 2011, 62, 584–598. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.R.; Moreira, J.; Pereira, D.; Pereira, S.; Antunes, J.; Palmeira, A.; Vasconcelos, V.; Pinto, M.; Correia-da-Silva, M.; Cidade, H. Potential of synthetic chalcone derivatives to prevent marine biofouling. Sci. Total Environ. 2018, 643, 98–106. [Google Scholar] [CrossRef]
- Cattò, C.; Dell’Orto, S.; Villa, F.; Villa, S.; Gelain, A.; Vitali, A.; Marzano, V.; Baroni, S.; Forlani, F.; Cappitelli, F. Unravelling the structural and molecular basis responsible for the anti-biofilm activity of zosteric acid. PLoS ONE 2015, 10, e0131519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, L.; Bartoli, F.; Fidanza, M.R.; Zurlo, F.; Marconi, E.; Gasperi, T.; Tuti, S.; Crociani, L.; Di Bartolomeo, E.; Caneva, G.; et al. Encapsulation of environmentally-friendly biocides in silica nanosystems for multifunctional coatings. Appl. Surf. Sci. 2020, 514, 145908. [Google Scholar] [CrossRef]
- Ruggiero, L.; Crociani, L.; Zendri, E.; El Habra, N.; Guerriero, P. Incorporation of the zosteric sodium salt in silica nanocapsules: Synthesis and characterization of new fillers for antifouling coatings. Appl. Surf. Sci. 2018, 439, 705–711. [Google Scholar] [CrossRef]
- Bartoli, F.; Zuena, M.; Sodo, A.; Caneva, G. The efficiency of biocidal silica nanosystems for the conservation of stone monuments: Comparative in vitro tests against epilithic green algae. Appl. Sci. 2021, 11, 6804. [Google Scholar] [CrossRef]
- Ruggiero, L.; Fidanza, M.R.; Iorio, M.; Tortora, L.; Caneva, G.; Ricci, M.A.; Sodo, A. Synthesis and characterization of TEOS coating added with innovative antifouling silica nanocontainers and TiO2 nanoparticles. Frontiers 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Zuena, M.; Ruggiero, L.; Della Ventura, G.; Bemporad, E.; Ricci, M.A.; Sodo, A. Effectiveness and compatibility of nanoparticle based multifunctional coatings on natural and man-made stones. Coatings 2021, 11, 480. [Google Scholar] [CrossRef]
- Haake, S.; Simon, S.; Favaro, M. The bologna cocktail-evaluation of consolidation treatments on monuments in france and italy after 20 years on natural aging. In Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, Stockholm, Sweden, 27 June–2 July 2004; pp. 423–430. [Google Scholar]
- Ruggiero, L.; Di Bartolomeo, E.; Gasperi, T.; Luisetto, I.; Talone, A.; Zurlo, F.; Peddis, D.; Ricci, M.A.; Sodo, A. Silica nanosystems for active antifouling protection: Nanocapsules and mesoporous nanoparticles in controlled release applications. J. Alloys Compd. 2019, 798, 144–148. [Google Scholar] [CrossRef]
- Xu, F.; Li, D.; Zhang, Q.; Zhang, H.; Xu, J. Effects of addition of colloidal silica particles on TEOS-based stone protection using n-octylamine as a catalyst. Prog. Org. Coat. 2012, 75, 429–434. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2-SiO2-PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Pinho, L.; Elhaddad, F.; Facio, D.S.; Mosquera, M.J. A novel TiO2—SiO2 nanocomposite converts a very friable stone into a self-cleaning building material. Appl. Surf. Sci. 2013, 275, 389–396. [Google Scholar] [CrossRef]
- Pinho, L.; Mosquera, M.J. Photocatalytic activity of TiO2-SiO2 nanocomposites applied to buildings: Influence of particle size and loading. Appl. Catal. B Environ. 2013, 134–135, 205–221. [Google Scholar] [CrossRef]
- Scherer, G.W.; Wheeler, G.S. Silicate consolidants for stone. Key Eng. Mater. 2008, 391, 1–25. [Google Scholar] [CrossRef]
- Sassoni, E.; Franzoni, E.; Pigino, B.; Scherer, G.W.; Naidu, S. Consolidation of calcareous and siliceous sandstones by hydroxyapatite: Comparison with a TEOS-based consolidant. J. Cult. Herit. 2013, 14, e103–e108. [Google Scholar] [CrossRef]
- NORMAL 43/93 Misure Colorimetriche di Superfici Opache (Italian Normative on Stone Material—Colorimetric Measurement of Opaque Surfaces); Commissione Beni Culturali UNI NORMAL: Roma, Italy, 1993.
- EN UNI 15801:2010 Conservation of Cultural Property—Test Methods—Determination of Water Absorption by Capillarity. 2010. Available online: https://standards.iteh.ai/catalog/standards/cen/06e6ae49-1d9a-4318-b79b-eae91a02091c/en-15801-2009 (accessed on 9 December 2009).
- DIN 52 615 Testing of Thermal Insulating Material. In Determination of Water Vapour Permeability of Construction and Insulating Materials; Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 1987.
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81A, 89. [Google Scholar] [CrossRef]
- Hobson, T. Parameters and Definitions for Roundness and Surface Measurements; Taylor Hobson Ltd.: Leicester, UK, 2000. [Google Scholar]
- Della Volpe, C.; Penati, A.; Peruzzi, R.; Siboni, S.; Toniolo, L.; Colombo, C. Combined effect of roughness and heterogeneity on contact angles: The case of polymer coating for stone protection. J. Adhes. Sci. Technol. 2000, 14, 273–299. [Google Scholar] [CrossRef]
- Alvarez de Buergo Ballester, M.; Fort González, R. Basic methodology for the assessment and selection of water-repellent treatments applied on carbonatic materials. Prog. Org. Coat. 2001, 43, 258–266. [Google Scholar] [CrossRef]
- Sasse, H.R.; Snethlage, R. Methods for the evaluation of stone conservation treatments. In Saving Our Cultural Heritage: The Conservation of Historic Stone Structure, Dahlem Workshop Reports; Baer, N.S., Snethlage, R., Eds.; Wiley & Sons: Chichester, UK, 1997; pp. 223–243. [Google Scholar]
- Delgado Rodrigues, J.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Tsakalof, A.; Manoudis, P.; Karapanagiotis, I.; Chryssoulakis, I.; Panayiotou, C. Assessment of synthetic polymeric coatings for the protection and preservation of stone monuments. J. Cult. Herit. 2007, 8, 69–72. [Google Scholar] [CrossRef]






| Nanocontainer | Morphology | Size (nm) | Loading Capability (Weight %) |
|---|---|---|---|
| NC | Spherical | 170 ± 20 (diameter) | 2.1 |
| MNP | Rods | 100–1000 (length) | 7.8 |
| Sample | Description | Techniques |
|---|---|---|
| NT | Untreated samples | SCA, CM, WAC, WVP, OSR, SEM, PT |
| Si_Control | Coating without nanoparticles | SCA, CM, WAC, WVP, OSR, SEM, PT |
| Si_TiO2–NC | Coating with TiO2 nanoparticles and loaded silica nanocapsules | SCA, CM, WAC, WVP, OSR, SEM, PT |
| Si_TiO2–MNP | Coating with TiO2 nanoparticles and loaded silica mesoporous nanoparticles | SCA, CM, WAC, WVP, OSR, SEM, PT |
| Si_TiO2 | Coating with only titanium nanoparticles | PT |
| Sample | Average SCA (°) | ||
|---|---|---|---|
| Si_Control | Si_TiO2-NC | Si_TiO2-MNP | |
| Brick | 125.7 ± 4.7 | 138.2 ± 5.1 | 137.6 ± 4.5 |
| Mortar | 127.7 ± 3.5 | 130.1 ± 5.5 | 124.1 ± 4.3 |
| Travertine | 118.9 ± 5.1 | 120.2 ± 5.1 | 130.3 ± 1.9 |
| Carrara Marble | 125.5 ± 4.2 | 142.1 ± 3.3 | 134.2 ± 5.1 |
| Sample | Reduction in CWAC (%) | ||
|---|---|---|---|
| Si_Control | Si_TiO2–NC | Si_TiO2–MNP | |
| Brick | 96.49 | 97.8 | 99.1 |
| Mortar | 98.97 | 98.9 | 99.5 |
| Travertine | 10.52 | 70.0 | 52.5 |
| Carrara marble | 46.25 | 47.8 | 68.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuena, M.; Ruggiero, L.; Caneva, G.; Bartoli, F.; Ventura, G.D.; Ricci, M.A.; Sodo, A. Assessment of Stone Protective Coatings with a Novel Eco-Friendly Encapsulated Biocide. Coatings 2021, 11, 1109. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11091109
Zuena M, Ruggiero L, Caneva G, Bartoli F, Ventura GD, Ricci MA, Sodo A. Assessment of Stone Protective Coatings with a Novel Eco-Friendly Encapsulated Biocide. Coatings. 2021; 11(9):1109. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11091109
Chicago/Turabian StyleZuena, Martina, Ludovica Ruggiero, Giulia Caneva, Flavia Bartoli, Giancarlo Della Ventura, Maria Antonietta Ricci, and Armida Sodo. 2021. "Assessment of Stone Protective Coatings with a Novel Eco-Friendly Encapsulated Biocide" Coatings 11, no. 9: 1109. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11091109