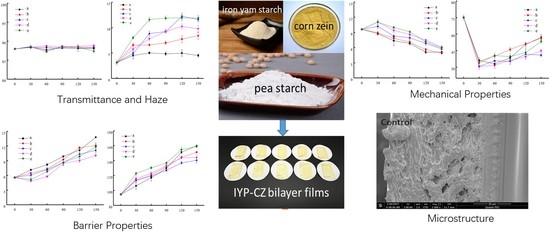
Effect of Storage Conditions on the Physicochemical Characteristics of Bilayer Edible Films Based on Iron Yam–Pea Starch Blend and Corn Zein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation and Storage
2.3. Color
2.4. Transmittance and Haze
2.5. Water Content
2.6. Water Vapor Permeability
2.7. Oxygen Permeability
2.8. Mechanical Properties
2.9. Scanning Electron Microscope (SEM)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Color
3.2. Transmittance and Haze
3.3. Water Content
3.4. Barrier Properties
3.5. Mechanical Properties
3.6. Scanning Electron Microscope (SEM)
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arifin, H.R.; Djali, M.; Nurhadi, B.; Hasim, S.A.; Hilmi, A.; Puspitasari, A.V. Improved properties of corn starch-based bio-nanocomposite film with different types of plasticizers reinforced by nanocrystalline cellulose. Int. J. Food Prop. 2022, 25, 509–521. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements- a review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.F. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Pirnia, M.; Shirani, K.; Yazdi, F.T.; Moratazavi, S.A.; Mohebbi, M. Characterization of antioxidant active biopolymer bilayer film based on gelatin-frankincense incorporated with ascorbic acid and Hyssopus officinalis essential oil. Food Chem. X 2022, 14, 100300. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Jin, Z.; Chen, F.; Lai, S.; Yang, H.S. Effect of chitosan coatings on the evolution of sodium carbonate-soluble pectin during sweet cherry softening under non-isothermal conditions. Int. J. Biol. Macromol. 2020, 154, 267–275. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, N.; Upadhyay, A.; Sethi, S.; Singh, A. Edible coating as postharvest management strategy for shelf-life extension of fresh tomato (Solanum lycopersicum L.): An overview. J. Food Sci. 2022, 87, 2256–2290. [Google Scholar] [CrossRef]
- Danyxa, P.H.; Carolina, M.J.; Alex, L.C.; Silvia, G. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar]
- Olaimat, A.N.; Sawalha, A.G.A.; Al-Nabulsi, A.A.; Osaili, T.; Al-Biss, B.A.; Ayyash, M.; Holley, R.A. Chitosan–ZnO nanocomposite coating for inhibition of Listeria monocytogenes on the surface and within white brined cheese. J. Food Sci. 2022, 87, 3151–3162. [Google Scholar] [CrossRef]
- Podshivalov, A.; Zakharova, M.; Glazacheva, E.; Uspenskaya, M. Gelatin/potato starch edible biocomposite films: Correlation between morphology and physical properties. Carbohydr. Polym. 2017, 157, 1162–1172. [Google Scholar] [CrossRef]
- Singh, T.P.; Chauhan, G.; Mendiratta, S.K.; Agrawal, R.K.; Arora, S.; Verma, A.K.; Rajkumar, V. In vitro antioxidant and antimicrobial activities of clove extract and its effectiveness in bio-composite film on storage stability of goat meat balls. J. Food Sci. 2022, 87, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Saberi, B.; Pristijono, P.; Golding, J.; Stathopoulos, C.; Scarlett, C.; Bowyer, M.; Vuong, Q. Characterization of rice starch-ι-carrageenan biodegradable edible film. Effect of stearic acid on the film properties. Int. J. Biol. Macromol. 2016, 93, 952–960. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.H.; Wang, K.; Xiao, J.D.; Liu, Y.W.; Zhao, Y.; Liu, A.J. Performance of high amylose starch-composited gelatin films influenced by gelatinization and concentration. Int. J. Biol. Macromol. 2017, 94, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Chowdhury, T. Heat sealing property of starch based self-supporting edible films. Food Packag. Shelf Life 2016, 9, 64–68. [Google Scholar] [CrossRef]
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind. Crops Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Peng, Y.; Yin, L.; Li, Y.F. Combined effects of lemon essential oil and surfactants on physical and structural properties of chitosan films. Int. J. Food Sci. Technol. 2013, 48, 44–50. [Google Scholar] [CrossRef]
- Piccirilli, G.N.; Soazo, M.; Pérez, L.M.; Delorenzi, N.J.; Verdini, R.A. Effect of storage conditions on the physicochemical characteristics of edible films based on whey protein concentrate and liquid smoke. Food Hydrocoll. 2019, 87, 221–228. [Google Scholar] [CrossRef]
- Leerahawong, A.; Tanaka, M.; Okazaki, E.; Osako, K. Stability of the physical properties of plasticized edible films from squid (Todarodes pacificus) mantle muscle during storage. J. Food Sci. 2012, 77, E159–E165. [Google Scholar] [CrossRef]
- Matsakidou, A.; Tsimidou, M.Z.; Kiosseoglou, V. Storage behavior of caseinate-based films incorporating maize germ oil bodies. Food Res. Int. 2019, 116, 1031–1040. [Google Scholar] [CrossRef]
- Osés, J.; Idoya, F.P.; Mendoza, M.; Maté, J.I. Stability of the mechanical properties of edible films based on whey protein isolate during storage at different relative humidity. Food Hydrocoll. 2009, 23, 125–131. [Google Scholar] [CrossRef]
- Famá, L.; Goyanes, S.; Gerschenson, L. Influence of storage time at normal temperature on the physicochemical properties of cassava starch films. Carbohydr. Polym. 2007, 70, 265–273. [Google Scholar] [CrossRef]
- Nuria, B.P.; Fernando, F.M.; Pilar, M. Jumbo squid (Dosidicus gigas) myofibrillar protein concentrate for edible packaging films and storage stability. LWT-Food Sci. Technol. 2014, 55, 543–550. [Google Scholar]
- Xiao, G.N.; Zhu, Y.B.; Wang, L.X.; You, Q.; Huo, P.; You, Y.R. Production and Storage of Edible Film Using Gellan Gum. Procedia Environ. Sci. 2011, 8, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef]
- Neira, L.M.; Martucci, J.F.; Stejskal, N.; Ruseckaite, R.A. Time-dependent evolution of properties of fish gelatin edible films enriched with carvacrol during storage. Food Hydrocoll. 2019, 94, 304–310. [Google Scholar] [CrossRef]
- Borneo, R.; Alba, N.; Aguirre, A. New films based on triticale flour: Properties and effects of storage time. J. Cereal Sci. 2016, 68, 82–87. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Storage-induced changes in functional properties of glycerol plasticized–soybean protein concentrate films produced by casting. Food Hydrocoll. 2015, 45, 247–255. [Google Scholar] [CrossRef]
- Schmid, M.; Merzbacher, S.; Müller, K. Time-dependent crosslinking of whey protein based films during storage. Mater. Lett. 2018, 215, 8–10. [Google Scholar] [CrossRef]
- Ket-On, A.; Pongmongkol, N.; Somwangthanaroj, A.; Janjarasskul, T.; Tananuwong, K. Properties and storage stability of whey protein edible film with spice powders. J. Food Sci. Technol. 2016, 53, 2933–2942. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Reichert, K.; Hammann, F.; Stäbler, A. Storage time-dependent alteration of molecular interaction–property relationships of whey protein isolate based films and coatings. J. Mater. Sci. 2015, 50, 4396–4404. [Google Scholar] [CrossRef]
- Leceta, I.; Peñalba, M.; Arana, P.; Guerrero, P.; de la Caba, K. Ageing of chitosan films: Effect of storage time on structure and optical, barrier and mechanical properties. Eur. Polym. J. 2015, 66, 170–179. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; López-Rubio, A.; Del-Valle, V.; Almenar, E.; Gavara, R. Mechanical and water barrier properties of glutenin films influenced by storage time. J. Agric. Food Chem. 2004, 52, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Matta, E.; Tavera-Quiroz, M.J.; Bertola, N. Isomalt-Plasticized Methylcellulose-Based Films as Carriers of Ascorbic Acid. Food Bioprocess Technol. 2020, 13, 2186–2199. [Google Scholar] [CrossRef]
- Friedrich, J.C.C.; Silva, O.A.; Faria, M.G.I.; Colauto, N.B.; Gazzin, Z.C.; Colauto, G.A.L.; Caetano, J.; Dragunski, D.C. Improved antioxidant activity of a starch and gelatin-based biodegradable coating containing Tetradenia riparia extract. Int. J. Biol. Macromol. 2020, 165, 1038–1046. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zheng, X.Q.; Liu, X.L.; Kopparapu, N.K.; Cong, W.S.; Deng, Y.P. Preparation of glycosylated zein and retarding effect on lipid oxidation of ground pork. Food Chem. 2017, 227, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.C.; Yin, S.W.; Yang, X.Q.; Tang, C.H.; Wen, S.H.; Chen, Z.; Xiao, B.J.; Wu, L.Y. Surface modification of sodium caseinate films by zein coatings. Food Hydrocoll. 2014, 36, 1–8. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Oromiehi, A.R. Biodegradable biocomposite films based on whey protein and zein: Barrier, mechanical properties and AFM analysis. Int. J. Biol. Macromol. 2008, 43, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Fund, T.; Isa, D.A.; Banu, O. Water vapor and oxygen-barrier performance of corn–zein coated polypropylene films. J. Food Eng. 2010, 96, 342–347. [Google Scholar]
- Dong, S.; Gao, A.; Xu, H.; Chen, Y. Effects of Dielectric Barrier Discharges (DBD) Cold PlasmaTreatment on Physicochemical and Structural Propertiesof Zein Powders. Food Bioprocess Technol. 2017, 10, 434–444. [Google Scholar] [CrossRef]
- Nagar, M.; Sharanagat, V.S.; Kumar, Y.; Singh, L. Development and characterization of elephant foot yam starch–hydrocolloids based edible packaging film: Physical, optical, thermal and barrier properties. J. Food Sci. Technol. 2020, 57, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Thakur, R.; Bhuyan, D.J.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarletta, C.J.; Stathopoulosc, C.E. Development of edible blend films with good mechanical and barrier properties from pea starch and guar gum. Starch Starke 2017, 69, 1–15. [Google Scholar] [CrossRef]
- Zuo, G.J.; Song, X.Y.; Chen, F.S.; Shen, Z.X. Physical and structural characterization of edible bilayer films made with zein and corn-wheat starch. J. Saudi Soc. Agric. Sci. 2017, 18, 324–331. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, J.; Zhao, J.; Bian, W.; Li, Y.; Wang, X.J. Adsorption behavior of modified Iron stick yam skin with Polyethyleneimine as a potential biosorbent for the removal of anionic dyes in single and ternary systems at low temperature. Bioresour. Technol. 2016, 222, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Wang, Y.Q. Development and characterization of edible bilayer films based on iron yam-pea starch blend and corn zein. Food Sci. Technol. 2021, 41, 684–694. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT-Food Sci. Technol. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Song, X.Y.; Cheng, L.M.; Tan, L. Edible iron yam and maize starch convenient food flavoring packaging films with lemon essential oil as plasticization. Food Sci. Technol. 2019, 39, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Erahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Aliabadi, S.S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT-Food Sci. Technol. 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Pérez, L.M.; Piccirilli, G.N.; Delorenzi, N.J.; Verdini, R.A. Effect of different combinations of glycerol and/or trehalose on physical and structural properties of whey protein concentrate-based edible films. Food Hydrocoll. 2016, 56, 352–359. [Google Scholar] [CrossRef]
- Korhonen, K.; Smolander, E.; Korhonen, O.; Ketolainen, J.; Laitinen, R. Effect of storage on the physical stability of thin polymethacrylate-perphenazine films. Eur. J. Pharm. Sci. 2017, 104, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ciannamea, E.M.; Espinosa, J.P.; Stefani, P.M.; Ruseckaite, R.A. Long-term stability of compression-molded soybean protein concentrate films stored under specific conditions. Food Chem. 2018, 243, 448–452. [Google Scholar] [CrossRef] [PubMed]






| Storage Conditions | Storage Time | |||||
|---|---|---|---|---|---|---|
| 30 d | 60 d | 90 d | 120 d | 150 d | ||
| L* | −18 °C | 94.47 ± 0.32 b,A | 94.31 ± 0.24 ab,A | 94.28 ± 0.31 a,A | 94.28 ± 0.54 ab,A | 93.77 ± 0.10 ab,B |
| −5 °C | 94.62 ± 0.14 b,A | 94.53 ± 0.40 b,A | 94.64 ± 0.22 b,B | 94.46 ± 0.56 b,A | 93.44 ± 0.54 a,A | |
| 25 °C, RH:43% | 94.67 ± 0.33 a,A | 94.47 ± 0.07 a,A | 94.22 ± 0.31 a,AB | 94.35 ± 0.59 a,A | 94.17 ± 0.31 a,B | |
| 25 °C, RH:54% | 94.75 ± 0.30 b,A | 94.33 ± 0.22 b,A | 94.33 ± 0.28 b,B | 94.37 ± 0.12 b,A | 93.75 ± 0.41 a,AB | |
| 25 °C, RH:65% | 94.44 ± 0.16 a,A | 94.34 ± 0.38 a,A | 94.29 ± 0.24 a,B | 94.06 ± 0.27 a,A | 94.08 ± 0.29 a,AB | |
| a* | −18 °C | −2.90 ± 0.12 a,A | −3.11 ± 0.20 a,A | −3.15 ± 0.04 a,A | −3.17 ± 0.15 a,A | −3.19 ± 0.19 a,A |
| −5 °C | −2.70 ± 0.10 bc,A | −2.76 ± 0.01 c,B | −2.88 ± 0.20 c,B | −3.02 ± 0.16 ab,A | −3.16 ± 0.13 a,A | |
| 25 °C, RH:43% | −2.63 ± 0.08 a,B | −2.66 ± 0.10 a,B | −2.70 ± 0.17 a,B | −2.72 ± 0.14 a,B | −2.72 ± 0.16 a,B | |
| 25 °C, RH:54% | −2.14 ± 0.07 b,C | −2.31 ± 0.13 ab,C | −2.30 ± 0.16 ab,C | −2.42 ± 0.13 ab,C | −2.63 ± 0.33 a,B | |
| 25 °C, RH:65% | −1.50 ± 0.18 c,D | −1.66 ± 0.15 bc,D | −1.69 ± 0.09 ab,D | −1.82 ± 0.15 bc,D | −2.05 ± 0.14 a,C | |
| b* | −18 °C | 17.37 ± 0.42 b,D | 17.07 ± 0.53 b,D | 16.87 ± 0.67 b,D | 16.66 ± 0.26 b,C | 15.50 ± 0.37 a,C |
| −5 °C | 16.59 ± 0.58 b,CD | 16.57 ± 0.84 b,D | 15.53 ± 0.40 ab,C | 14.87 ± 0.48 a,B | 14.65 ± 0.71 a,C | |
| 25 °C, RH:43% | 16.01 ± 0.52 a,C | 14.98 ± 0.72 a,C | 14.95 ± 0.61 a,B | 14.91 ± 0.78 a,B | 14.66 ± 0.87 a,BC | |
| 25 °C, RH:54% | 14.10 ± 0.82 a,B | 14.06 ± 0.33 a,B | 13.49 ± 0.79 a,B | 13.32 ± 0.84 a,A | 13.32 ± 0.78 a,AB | |
| 25 °C, RH:65% | 12.47 ± 0.55 a,A | 12.13 ± 0.54 a,A | 12.01 ± 0.87 a,A | 11.96 ± 0.45 a,A | 11.57 ± 1.01 a,A | |
| WI | −18 °C | 81.54 ± 0.49 a,A | 81.69 ± 0.57 a,A | 81.74 ± 0.73 a,A | 82.05 ± 0.21 a,A | 83.18 ± 0.38 a,A |
| −5 °C | 82.32 ± 0.55 a,AB | 82.34 ± 0.72 a,A | 83.35 ± 0.45 ab,B | 83.43 ± 0.49 b,B | 84.04 ± 0.68 ab,A | |
| 25 °C, RH:43% | 82.92 ± 0.44 a,B | 83.71 ± 0.65 a,B | 83.80 ± 0.50 a,BC | 83.87 ± 0.76 a,B | 83.99 ± 0.92 a,AB | |
| 25 °C, RH:54% | 84.66 ± 0.85 a,C | 84.80 ± 0.24 a,C | 85.04 ± 0.65 a,C | 85.17 ± 0.71 a,C | 85.35 ± 0.62 a,B | |
| 25 °C, RH:65% | 86.17 ± 0.54 a,D | 86.50 ± 0.55 a,D | 86.51 ± 0.76 a,D | 86.68 ± 0.38 a,C | 86.96 ± 1.02 a,C | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X. Effect of Storage Conditions on the Physicochemical Characteristics of Bilayer Edible Films Based on Iron Yam–Pea Starch Blend and Corn Zein. Coatings 2022, 12, 1524. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12101524
Song X. Effect of Storage Conditions on the Physicochemical Characteristics of Bilayer Edible Films Based on Iron Yam–Pea Starch Blend and Corn Zein. Coatings. 2022; 12(10):1524. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12101524
Chicago/Turabian StyleSong, Xiaoyong. 2022. "Effect of Storage Conditions on the Physicochemical Characteristics of Bilayer Edible Films Based on Iron Yam–Pea Starch Blend and Corn Zein" Coatings 12, no. 10: 1524. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12101524