Calcium Phosphate Loaded Biopolymer Composites—A Comprehensive Review on the Most Recent Progress and Promising Trends
Abstract
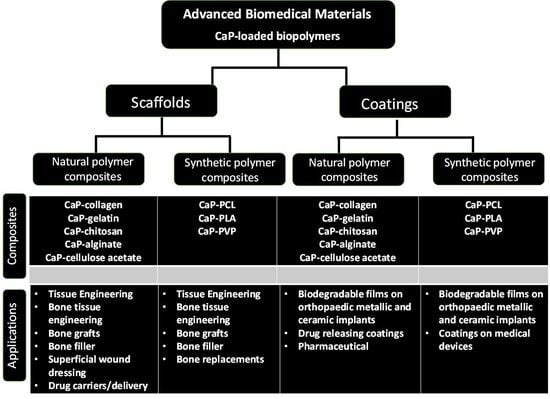
:1. Introduction
2. CaP Containing Biopolymer Composites in Bone Tissue Engineering
2.1. Composites Prepared with Biopolymers from a Natural Source
2.1.1. Collagen-Based Composites
2.1.2. Gelatin-Based Bioceramic Composites
2.1.3. Chitosan-Based Bioceramic Composites
2.1.4. Alginate-Based Bioceramic Composites
2.1.5. Cellulose-Based Bioceramic Composites
3. Composites Prepared with Synthetic Biopolymers
3.1. Polylactic Acid (PLA) Composites (Biomass-Based)
3.2. Polyvinylpyrrolidone (PVP) Composites (Petroleum-Based)
3.3. Polycaprolactone (PCL) Composites (Petroleum-Based)
4. Blended Polymer Composites
4.1. Synthetic-Natural Blended Polymer Composites
4.2. Synthetic/Synthetic Blended Polymer Composites
5. Future Perspectives and Possible Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, A.; Alamry, K.A.; Asiri, A.M. Multifunctional Biopolymers-Based Composite Materials for Biomedical Applications: A Systematic Review. Nanomater. Polym. Chem. Sel. 2021, 6, 154–176. [Google Scholar] [CrossRef]
- Barinov, S.M. Calcium phosphate-based ceramic and composite materials for medicine. Russ. Chem. Rev. 2010, 79, 13–29. [Google Scholar] [CrossRef]
- Shuai, C.; Yu, L.; Feng, P.; Gao, C.; Peng, S. Interfacial reinforcement in bioceramic/biopolymer composite bone scaffold: The role of coupling agent. Colloids Surf. B Biointerfaces 2020, 193, 111083. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.S.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, synthetic and commercially-available biopolymers used to regenerate tendons and ligaments. Bioact. Mater. 2023, 19, 179–197. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V. Implantable Devices: Issues and Challenges. Electronics 2013, 2, 1–34. [Google Scholar] [CrossRef]
- Hayakawa, S.; Tsuru, K.; Osaka, A. Chapter 3—The microstructure of bioceramics and its analysis. In Bioceramics and Their Clinical Applications; Tadashi, K., Ed.; Woodhead Publishing: Cambridge, England, 2008; pp. 53–77. [Google Scholar]
- Grebņevs, V.; Leśniak-Ziółkowska, K.; Wala, M.; Dulski, M.; Altundal, S.; Dutovs, A.; Avotiņa, L.; Erts, D.; Viter, R.; Vīksna, A.; et al. Modification of physicochemical properties and bioactivity of oxide coatings formed on Ti substrates via plasma electrolytic oxidation in crystalline and amorphous calcium phosphate particle suspensions. Appl. Surf. Sci. 2022, 598, 153793. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering 2022, 9, 728. [Google Scholar] [CrossRef]
- Liu, H.; Webster, T.J. Mechanical properties of dispersed ceramic nanoparticles in polymer composites for orthopedic applications. Int. J. Nanomed. 2010, 5, 299–313. [Google Scholar]
- Furko, M.; Horváth, Z.E.; Mihály, J.; Balázsi, K.; Balázsi, C. Comparison of the Morphological and Structural Characteristic of Bioresorbable and Biocompatible Hydroxyapatite-Loaded Biopolymer Composites. Nanomaterials 2021, 11, 3194. [Google Scholar] [CrossRef]
- Rivero, P.J.; Redin, D.M.; Rodríguez, R.J. Electrospinning: A Powerful Tool to Improve the Corrosion Resistance of Metallic Surfaces Using Nanofibrous Coatings. Metals 2020, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Vishwakarma, V.; Kaliaraj, G.S.; Kirubaharan, A.M.K. Advanced Alloys and Coatings for Bioimplants. Coatings 2022, 12, 1525. [Google Scholar] [CrossRef]
- Ali, A.A.; Barakat, N.A.M.; Lim, J.K. Influence of electrospinning and dip-coating techniques on the degradation and cytocompatibility of Mg-based alloy 2013. Colloids Surf. A Physicochem. Eng. Asp. 2013, 420, 37–45. [Google Scholar]
- Esposti, M.D.; Changizi, M.; Salvatori, R.; Chiarini, L.; Cannillo, V.; Morselli, D.; Fabbri, P. Comparative Study on Bioactive Filler/Biopolymer Scaffolds for Potential Application in Supporting Bone Tissue Regeneration. ACS Appl. Polym. Mater. 2022, 4, 4306–4318. [Google Scholar] [CrossRef]
- Shakil, U.A.; Abu Hassan, S.B.; Yahya, M.Y.; Rejab, M.R. A focused review of short electrospun nanofiber preparation techniques for composite reinforcement. Nanotechnol. Rev. 2022, 11, 1991–2014. [Google Scholar] [CrossRef]
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.; Socol, G. Degradation Behavior of Polymers Used as Coating Materials for Drug Delivery—A Basic Review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 2011, 1, 3–56. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, C. Morphological, chemical, and biological investigation of ionic substituted, pulse current deposited calcium phosphate coatings. Materials 2020, 13, 4690. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Hurt, A.P.; Kotha, A.K.; Coleman, N.J. Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses. J. Funct. Biomater. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Tattanon, T.; Arpornmaeklong, P.; Ummartyotin, S.; Pongprayoon, T. Hydrothermal Synthesis of Biphasic Calcium Phosphate from Cuttlebone Assisted by the Biosurfactant L-rhamnose Monohydrate for Biomedical Materials. ChemEngineering 2021, 5, 88. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Sipaut, C.S.; Nasri, M.; Arshad, S.E.B. Hydrothermal synthesis of hydroxyapatite powders using Response Surface Methodology (RSM). PLoS ONE 2021, 16, e0251009. [Google Scholar] [CrossRef] [PubMed]
- Sterner, P.; Biernat, M. The Synthesis of Hydroxyapatite by Hydrothermal Process with Calcium Lactate Pentahydrate: The Effect of Reagent Concentrations, pH, Temperature, and Pressure. Bioinorg. Chem. Appl. 2022, 2022, 3481677. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Negrila, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D. Development of Zinc-Doped Hydroxyapatite by Sol-Gel Method for Medical Applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef]
- Daltin, A.L.; Beaufils, S.; Rouillon, T.; Millet, P.; Chopart, J.P. Calcium phosphate powder synthesis by out-of-phase pulsed sonoelectrochemistry. Ultrason. Sonochem. 2019, 58, 104662. [Google Scholar] [CrossRef]
- Dinda, S.; Bhagavatam, A.; Alrehaili, H.; Dinda, G.P. Mechanochemical Synthesis of Nanocrystalline Hydroxyapatite from Ca(H2PO4)2.H2O, CaO, Ca(OH)2, and P2O5 Mixtures. Nanomaterials 2020, 10, 2232. [Google Scholar] [CrossRef]
- Lukina, Y.; Kotov, S.; Bionyshev-Abramov, L.; Serejnikova, N.; Chelmodeev, R.; Fadeev, R.; Toshev, O.; Tavtorkin, A.; Ryndyk, M.; Smolentsev, D.; et al. Low-Temperature Magnesium Calcium Phosphate Ceramics with Adjustable Resorption Rate. Ceramics 2023, 6, 168–194. [Google Scholar] [CrossRef]
- Otsuka, M.; Saito, H.; Sasaki, T. Analytical Evaluation of Wet and Dry Mechanochemical Syntheses of Calcium-Deficient Hydroxyapatite Containing Zinc Using X-ray Diffractometry and Near-Infrared Spectroscopy. Pharmaceutics 2022, 14, 2105. [Google Scholar] [CrossRef]
- Sun, L.; Chow, L.C.; Frukhtbeyn, S.A.; Bonevich, J.E. Preparation and Properties of Nanoparticles of Calcium Phosphates With Various Ca/P Ratios. J. Res. Natl. Inst. Stand. Technol. 2010, 115, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Itatani, K.; Iwafune, K.; Howell, F.S.; Aizawa, M. Preparation of various calcium-phosphate powders by ultrasonic spray freeze-drying technique. Mater. Res. Bull. 2000, 35, 575–585. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on Ionic Substitutions in Hydroxyapatite Thin Films: Towards Complete Biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Vidotto, M.; Grego, T.; Petrović, B.; Somers, N.; Antonić Jelić, T.; Kralj, D.; Matijaković Mlinarić, N.; Leriche, A.; Dutour Sikirić, M.; Erceg, I.; et al. A Comparative EPR Study of Non-Substituted and Mg-Substituted Hydroxyapatite Behaviour in Model Media and during Accelerated Ageing. Crystals 2022, 12, 297. [Google Scholar] [CrossRef]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Czömpöly, O.; Balázsi, K.; Balázsi, C. Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites. Int. J. Mol. Sci. 2022, 23, 15737. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Sulyok, A.; Mihály, J.; Balázsi, C. Preparation and morphological investigation on bioactive ion-modified carbonated hydroxyapatite-biopolymer composite ceramics as coatings for orthopedic implants. Ceram. Int. 2022, 48, 760–768. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, C. Calcium phosphate based bioactive ceramic layers on implant materials preparation, properties, and biological performance. Coatings 2020, 10, 823. [Google Scholar] [CrossRef]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M. Tecklenburg, Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Khan, A.S.; Awais, M. Low-Cost Deposition of Antibacterial Ion-Substituted Hydroxyapatite Coatings onto 316L Stainless Steel for Biomedical and Dental Applications. Coatings 2020, 10, 880. [Google Scholar] [CrossRef]
- Jun Wu, J.; Ueda, K.; Narushima, T. Fabrication of Ag and Ta co-doped amorphous calcium phosphate coating films by radiofrequency magnetron sputtering and their antibacterial activity. Mater. Sci. Eng. C 2020, 109, 110599. [Google Scholar]
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; Sglavo, V.M. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials 2021, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Dorcioman, G.; Grumezescu, V. A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards. Nanomaterials 2021, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-F.; Lee, M.-H.; Thomas, J.L.; Li, J.-A.; Wu, S.-C.; Hsu, H.-C.; Lin, H.-Y. Porous Biphasic Calcium Phosphate Granules from Oyster Shell Promote the Differentiation of Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2021, 22, 9444. [Google Scholar] [CrossRef] [PubMed]
- Balazsi, C.; Kövér, Z.; Horváth, E.; Németh, C.; Kasztovszky, Z.; Kurunczi, S.; Wéber, F. Examination of Calcium-Phosphates Prepared from Eggshell. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2007; Volume 537, pp. 105–112. [Google Scholar]
- Kalbarczyk, M.; Szcześ, A.; Kantor, I.; May, Z.; Sternik, D. Synthesis and Characterization of Calcium Phosphate Materials Derived from Eggshells from Different Poultry with and without the Eggshell Membrane. Materials 2022, 15, 934. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Cionita, T.; Lai, Y.L.; Fitriyana, D.F.; Siregar, J.P.; Bayuseno, A.P.; Nugraha, F.W.; Muhamadin, R.C.; Irawan, A.P.; Hadi, A.E. Characterization of PLA/PCL/Green Mussel Shells Hydroxyapatite (HA) Biocomposites Prepared by Chemical Blending Methods. Materials 2022, 15, 8641. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Akinwunmi, F.; Sodiya, E.F.; Alayande, S.O.; Ogundiran, A.A.; Ajayi, G.O. Biogenic preparation of biphasic calcium phosphate powder from natural source of snail shells: Bioactivity study. SN Appl. Sci. 2022, 4, 144. [Google Scholar] [CrossRef]
- Mocanu, A.C.; Stan, G.E.; Maidaniuc, A.; Miculescu, M.; Antoniac, I.V.; Ciocoiu, R.C.; Voicu, Ș.I.; Mitran, V.; Cîmpean, A.; Miculescu, F. Naturally-Derived Biphasic Calcium Phosphates through Increased Phosphorus-Based Reagent Amounts for Biomedical Applications. Materials 2019, 12, 381. [Google Scholar] [CrossRef]
- Hussin, M.S.F.; Abdullah, H.Z.; Idris, M.I.; Wahap, M.A.A. Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, M.; Taira, M.; Sawada, T.; Hachinohe, Y.; Hatakeyama, W.; Takafuji, K.; Tekemoto, S.; Kondo, H. Preparation of Collagen/Hydroxyapatite Composites Using the Alternate Immersion Method and Evaluation of the Cranial Bone-Forming Capability of Composites Complexed with Acidic Gelatin and b-FGF. Materials 2022, 15, 8802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Lou, Y.Y.; Li, T.H.; Liu, B.Z.; Chen, K.; Zhang, D.; Li, T. Cross-linking methods of type I collagen-based scaffolds for cartilage tissue engineering. Am. J. Transl. Res. 2022, 14, 146–1159. [Google Scholar]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2022, 10, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.-A.; Benítez-García, J.A.; Osorio, R. Differential biodegradation kinetics of collagen membranes for bone regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Huang, S.-S.; Yu, W.-X.; Hsu, Y.-W.; Hsu, F.-Y. Collagen scaffolds containing hydroxyapatite-CaO fiber fragments for bone tissue engineering. Polymers 2020, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, J.; Fan, D. Fabrication of high-strength and porous hybrid scaffolds based on nano-hydroxyapatite and human-like collagen for bone tissue regeneration. Polymers 2020, 12, 61. [Google Scholar] [CrossRef]
- Heinemann, S.; Coradin, T.; Worch, H.; Wiesmann, H.P.; Hanke, T. Possibilities and Limitations of preparing silica/collagen/hydroxyapatite composite xerogels as load-bearing biomaterials. Compos. Sci. Technol. 2011, 71, 1873–1880. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hussein, E.A.; Elzatahry, Á.A. Recent overviews in functional polymer composites for biomedical applications. Polymers 2018, 10, 739. [Google Scholar] [CrossRef]
- Saeidi, N.; Sander, E.A.; Zareian, R.; Ruberti, J.W. Production of highly aligned collagen lamellae by combining shear force and thin film confinement. Acta Biomater. 2011, 7, 2437–2447. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q.; Li, Z. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215. [Google Scholar]
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. An update on hydroxyapatite/collagen composites: What is there left to say about these bioinspired materials? J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Sobczak-Kupiec, A.; Drabczyk, A.; Florkiewicz, W.; Głąb, M.; Kudłacik-Kramarczyk, S.; Słota, D.; Tomala, A.; Tyliszczak, B. Review of the Applications of Biomedical Compositions Containing Hydroxyapatite and Collagen Modified by Bioactive Components. Materials 2021, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Kikuchi, M.; Takakuda, K.; Nagaoka, K.; Koyama, Y.; Tanaka, J.; Shinomiya, K. Implantation study of a novel hydroxyapatite/collagen (HAp/col) composite into weight-bearing sites of dogs. J. Biomed. Mater. Res. 2002, 63, 507–515. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef]
- Bartmanski, M.; Rosciszewska, M.; Wekwejt, M.; Ronowska, A.; Nadolska-Dawidowska, M.; Mielewczyk-Gryn, A. Properties of New Composite Materials Based on Hydroxyapatite Ceramic and Cross-Linked Gelatin for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9083. [Google Scholar] [CrossRef]
- Thiyagarajan, P.; Shanmugharaj, A.M.; Alagesan, T.; Padmapriya, A.; Kalaivani, R.A. Thermal Degradation Process and Kinetics of Gelatin/HAP Composites. ECS Trans. 2022, 107, 16507–16517. [Google Scholar]
- Hossan, J.; Gafur, M.A.; Kadir, M.R.; Karim, M.M. Preparation and Characterization of Gelatin-Hydroxyapatite Composite for Bone Tissue Engineering. Int. J. Eng. Technol. IJET-IJENS 2014, 14, 24–32. [Google Scholar]
- Salama, A. Recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int. J. Biol. Macromol. 2021, 178, 240–252. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Matei, C.E.; Socol, G.; Chifiriuc, M.C.; Bleotu, C.; Grossin, D.; Brouillet, F.; et al. Composite Drug Delivery System Based on Amorphous Calcium Phosphate–Chitosan: An Efficient Antimicrobial Platform for Extended Release of Tetracycline. Pharmaceutics 2021, 13, 1659. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A Review on Chitosan's Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Sergi, R.; Bellucci, D.; Salvatori, R.; Anesi, A.; Cannillo, V. Chitosan-Based Bioactive Glass Gauze. Materials 2020, 13, 2819. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Wu, B.; Su, Y.; Sun, T.; Guo, X. Recent Advances in the Application of Natural and Synthetic Polymer-Based Scaffolds in Musculoskeletal Regeneration. Polymers 2022, 14, 4566. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Nanostructured hydroxyapatite-chitosan composite biomaterial for bone tissue engineering. Adv. Mater. Res. 2012, 584, 212–216. [Google Scholar] [CrossRef]
- Shen, X.Y.; Chen, L.; Cai, X.A.; Tong, T.; Tong, H.; Hu, J.M. A Novel method for the fabrication of homogeneous hydroxyapatite-collagen nanocomposite and nanocomposite scaffold with hierarchical porosity. J. Mater. Sci. Mater. Med. 2011, 22, 299–305. [Google Scholar] [CrossRef]
- Sakthivel, P.; Ragu, A. Synthesis and characterization of nano hydroxyapatite with polymer matrix nano composite for biomedical applications. Int. J. Chem. Environ. Biol. Sci. 2015, 3, 2320–4087. [Google Scholar]
- Zhao, Y.; Zhao, S.; Ma, Z.; Ding, C.; Chen, J.; Li, J. Chitosan-Based Scaffolds for Facilitated Endogenous Bone Re-Generation. Pharmaceuticals 2022, 15, 1023. [Google Scholar] [CrossRef]
- Fedotov, A.Y.; Barinov, S.M.; Ievlev, V.M.; Sirotinkin, V.P.; Soldatenko, S.A.; Komlev, V.S. Formation of composite scaffolds based on chitosan and calcium phosphate. Dokl. Chem. 2016, 469, 215–218. [Google Scholar] [CrossRef]
- Kjalarsdóttir, L.; Dýrfjörd, A.; Dagbjartsson, A.; Laxdal, E.H.; Örlygsson, G.; Gíslason, J.; Einarsson, J.M.; Ng, C.-H.; Jónsson, H. Bone remodeling effect of a chitosan and calcium phosphate-based composite. Regen. Biomater. 2019, 6, 241–247. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Cao, W.; Gong, Y.; Zhao, N.; Zhang, X. Preparation and characterization of nano-hydroxyapatite/chitosan composite scaffolds. J. Biomed. Mater. Res. Part A 2005, 75, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.H.; Abdeltawab, N.F.; Awad, G.A.S. Chitosan-Calcium Phosphate Composite Scaffolds for Control of Post-operative Osteomyelitis: Fabrication, characterization, and in vitro–in vivo evaluation. Carbohydr. Polym. 2020, 244, 116482. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Krebs, M.D. Tunable chitosan-calcium phosphate composites as cell-instructive dental pulp capping agents. J. Biomater. Sci. Polym. Ed. 2021, 11, 1450–1465. [Google Scholar] [CrossRef] [PubMed]
- Örlygsson, G.; Laxdal, E.H.; Kárason, S.; Dagbjartsson, A.; Gunnarsson, E.; Ng, C.H.; Einarsson, J.M.; Gíslason, J.; Jónsson, H., Jr. Mineralization in a Critical Size Bone-Gap in Sheep Tibia Improved by a Chitosan-Calcium Phosphate-Based Composite as Compared to Predicate Device. Materials 2022, 15, 838. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Ribeiro, N.; Nunes, C.M.M.; Rodrigues, A.F.M.; Sousa, A.; Olhero, S.M. Toughening robocast chitosan/biphasic calcium phosphate composite scaffolds with silk fibroin: Tuning printable inks and scaffold structure for bone regeneration. Biomater. Adv. 2022, 134, 112690. [Google Scholar] [CrossRef]
- Lacan, I.; Moldovan, M.; Sarosi, C.; Ardelean, I. Chitosan Effect on Hardening Dynamics of Calcium Phosphate Cement: Low-Field NMR Relaxometry Investigations. Polymers 2022, 14, 3042. [Google Scholar] [CrossRef]
- Said, H.A.; Noukrati, H.; Ben Youcef, H.; Bayoussef, A.; Oudadesse, H.; Barroug, A. Mechanical Behavior of Hydroxyapatite-Chitosan Composite: Effect of Processing Parameters. Minerals 2021, 11, 213. [Google Scholar] [CrossRef]
- Iqbal, N.; Braxton, T.M.; Anastasiou, A.; Raif, E.M.; Chung, C.K.Y.; Kumar, S.; Giannoudis, P.V.; Jha, A. Dicalcium Phosphate Dihydrate Mineral Loaded Freeze-Dried Scaffolds for Potential Synthetic Bone Applications. Materials 2022, 15, 6245. [Google Scholar] [CrossRef]
- Teng, S.; Lee, E.; Yoon, B.; Shin, D.; Kim, H.; Oh, J. Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J. Biomed. Mater. Res. Part A 2009, 88, 569–580. [Google Scholar] [CrossRef]
- Zanca, C.; Patella, B.; Capuana, E.; Lopresti, F.; Brucato, V.; Carfì Pavia, F.; La Carrubba, V.; Inguanta, R. Behavior of Calcium Phosphate–Chitosan–Collagen Composite Coating on AISI 304 for Orthopedic Applications. Polymers 2022, 14, 5108. [Google Scholar] [CrossRef]
- Zarif, M.E.; Yehia-Alexe, S.A.; Bita, B.; Negut, I.; Locovei, C.; Groza, A. Calcium Phosphates–Chitosan Composite Layers Obtained by Combining Radio-Frequency Magnetron Sputtering and Matrix-Assisted Pulsed Laser Evaporation Techniques. Polymers 2022, 14, 5241. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2011, 22, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Lin, H.R.; Yeh, Y.J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: Preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 52–65. [Google Scholar] [CrossRef]
- Gu, Y.; Bai, Y.; Zhang, D. Osteogenic stimulation of human dental pulp stem cells with a novel gelatin-hydroxyapatite-tricalcium phosphate scaffold. J. Biomed. Mater. Res. A 2018, 106, 1851–1861. [Google Scholar] [CrossRef]
- Bjørnøy, S.H.; Bassett, D.C.; Ucar, S.; Andreassen, J.P.; Sikorski, P. Controlled mineralisation and recrystallisation of brushite within alginate hydrogels. Biomed. Mater. 2016, 11, 015013. [Google Scholar] [CrossRef]
- Sancilio, S.; Gallorini, M.; Di Nisio, C.; Marsich, E.; Di Pietro, R.; Schweikl, H.; Cataldi, A. Alginate/Hydroxyapatite-Based Nanocomposite Scaffolds for Bone Tissue Engineering Improve Dental Pulp Biomineralization and Differentiation. Stem Cells Int. 2018, 2018, 9643721. [Google Scholar] [CrossRef] [Green Version]
- You, F.; Chen, X.; Cooper, D.M.L.; Chang, T.; Eames, B.F. Homogeneous hydroxyapatite/alginate composite hydrogel promotes calcified cartilage matrix deposition with potential for three-dimensional bioprinting. Biofabrication 2019, 11, 015015. [Google Scholar] [CrossRef]
- Sikkema, R.; Keohan, B.; Zhitomirsky, I. Alginic Acid Polymer-Hydroxyapatite Composites for Bone Tissue Engineering. Polymers 2021, 13, 3070. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Y.; Zhang, J.; Bao, S.; Xian, L.; Dong, X.; Zheng, W.; Li, Y.; Gao, H.; Zhou, W. Bioactive and biocompatible macroporous scaffolds with tunable performances prepared based on 3D printing of the pre-crosslinked sodium alginate/hydroxyapatite hydrogel ink. Macromol. Mater. Eng. 2019, 304, 1800698. [Google Scholar] [CrossRef]
- Sancilio, S.; Marsich, E.; Schweikl, H.; Cataldi, A.; Gallorini, M. Redox control of IL-6-mediated dental pulp stem-cell differentiation on alginate/hydroxyapatite biocomposites for bone ingrowth. Nanomaterials 2019, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Sumayya, A.S.; Muraleedhara Kurup, G. Marine macromolecules cross-linked hydrogel scaffolds as physiochemically and biologically favorable entities for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Sukhodub, L.F.; Sukhodub, L.B.; Litsis, O.; Prylutskyy, Y. Synthesis and characterization of hydroxyapatite-alginate nanostructured composites for the controlled drug release. Mater. Chem. Phys. 2018, 217, 228–234. [Google Scholar] [CrossRef]
- Sangeetha, K.; Roy, A.; Singh, S.; Lee, B.; Kumta, P.N. Novel alginate-based coatings on Mg alloys. Mater. Sci. Eng. B 2011, 176, 1703–1710. [Google Scholar]
- Gholizadeh, B.S.; Buazar, F.; Hosseini, S.M.; Mousavi, S.M. Enhanced antibacterial activity, mechanical and physical properties of alginate/hydroxyapatite bionanocomposite film. Int. J. Biol. Macromol. 2018, 116, 786–792. [Google Scholar] [CrossRef]
- Cheong, M.; Zhitomirsky, I. Electrodeposition of alginic acid and composite films. Colloids Surf. A 2008, 328, 73–78. [Google Scholar] [CrossRef]
- Kollath, V.O.; Chen, Q.; Mullens, S.; Luyten, J.; Traina, K.; Boccaccini, A.R.; Cloots, R. Electrophoretic deposition of hydroxyapatite and hydroxyapatite–alginate on rapid prototyped 3D Ti6Al4V scaffolds. J. Mater. Sci. 2016, 51, 2338–2346. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Warcaba, M.; Cieniek, Ł.; Sitarz, M.; Gajewska, M.; Boccaccini, A.R. Hydroxyapatite/sodium alginate coatings electrophoretically deposited on titanium substrates: Microstructure and properties. Appl. Surf. Sci. 2021, 540, 148353. [Google Scholar] [CrossRef]
- Huang, C.; Dong, H.; Zhang, Z.; Bian, H.; Yong, Q. Procuring the Nano-Scale Lignin in Prehydrolyzate as Ingredient to Prepare Cellulose Nanofibril Composite Film with Multiple Functions. Cellulose 2020, 27, 9355–9370. [Google Scholar] [CrossRef]
- Huang, C.; Xu, C.; Meng, X.; Wang, L.; Zhou, X. Editorial: Isolation, modification, and characterization of the constituents (cellulose, hemicellulose, lignin; et al.) in biomass and their bio-based applications. Front. Bioeng. Biotechnol. 2022, 10, 866531. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhao, K.; Yu, D.G.; Zheng, X.; Huang, C. Advances in Biosensing and Environmental Monitoring Based on Electrospun Nanofibers. Adv. Fiber Mater. 2022, 4, 404–435. [Google Scholar] [CrossRef]
- Gupta, B.; Mishra, V.; Gharat, S.; Momin, M.; Omri, A. Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems. Pharmaceuticals 2021, 14, 1201. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium Hydroxyapatite Microfibrillated Cellulose Composite as a Potential Adsorbent for the Removal of Cr(VI) from Aqueous Solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
- Tabaght, F.E.; Azzaoui, K.; Elidrissi, A.; Hamed, O.; Mejdoubi, E.; Jodeh, S.; Akartasse, N.; Lakrat, M.; Lamhamdi, A. New Nanostructure Based on Hydroxyapatite Modified Cellulose for Bone Substitute, Synthesis, and Characterization. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 437–448. [Google Scholar] [CrossRef]
- Sivasankari, S.; Kalaivizhi, R.; Gowriboy, N.; Ganesh, M.R.; Shazia Anjum, M. Hydroxyapatite Integrated with Cellulose Acetate/polyetherimide Composite Membrane for Biomedical Applications. Polym. Compos. 2021, 42, 5512–5526. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Stoica, A.E.; Ene, D.-I.; Vasile, B.S.; Holban, A.M.; Neacsu, I.A. In Situ and Ex Situ Designed Hydroxyapatite: Bacterial Cellulose Materials with Biomedical Applications. Materials 2020, 13, 4793. [Google Scholar] [CrossRef]
- Chen, C.; Qian, J.; Chen, H.; Zhang, H.; Yang, L.; Jiang, X.; Zhang, X.; Li, X.; Ma, J.; Sun, D. Molecular Origin of the Biologically Accelerated Mineralization of Hydroxyapatite on Bacterial Cellulose for More Robust Nanocomposites. Nano Lett. 2021, 21, 10292–10300. [Google Scholar] [CrossRef]
- Palaveniene, A.; Tamburaci, S.; Kimna, C.; Glambaite, K.; Baniukaitiene, O.; Tihminlioğlu, F.; Liesiene, J. Osteoconductive 3D Porous Composite Scaffold from Regenerated Cellulose and Cuttlebone-Derived Hydroxyapatite. J. Biomater. Appl. 2019, 33, 876–890. [Google Scholar] [CrossRef]
- Pieper, C.M.; da Rosa, W.L.; Lund, R.G.; da Silva, A.F.; Piva, E.; Salas, M.M.; Maron, G.K.; Bomio, M.; Motta, F.V.; Carreño, N.L. Biofilms of Cellulose and Hydroxyapatite Composites: Alternative Synthesis Process. J. Bioact. Compat. Polym. 2020, 35, 469–478. [Google Scholar] [CrossRef]
- Gao, F.; Zeng, D.; Liu, H.; Qin, R.; Zhang, J.; Chen, Y.; Wang, W.; Peng, C.; Li, M.; Li, Q.; et al. Porous Cellulose Microspheres Coated in One Step with a Polydopamine Suspension of Hydroxyapatite for Bone Tissue Engineering. Cellulose 2022, 29, 1955–1967. [Google Scholar] [CrossRef]
- Shi, R.J.; Lang, J.Q.; Wang, T.; Zhou, N.; Ma, M.G. Fabrication, Properties, and Biomedical Applications of Calcium-Containing Cellulose-Based Composites. Front. Bioeng. Biotechnol. 2022, 10, 937266. [Google Scholar] [CrossRef] [PubMed]
- Abdelraof, M.; Farag, M.M.; Al-Rashidy, Z.M.; Ahmed, H.Y.A.; El-Saied, H.; Hasanin, M.S. Green Synthesis of Bioactive Hydroxyapatite/Cellulose Composites from Food Industrial Wastes. J. Inorg. Organomet. Polym. 2022, 32, 4614–4626. [Google Scholar] [CrossRef]
- Elsayed, M.T.; Hassan, A.A.; Abdelaal, S.A.; Taher, M.M.; Ahmed, M.K.; Shoueir, K.R. Morphological, antibacterial, and cell attachment of cellulose acetate nanofibers containing modified hydroxyapatite for wound healing utilizations. J. Mater. Res. Technol. 2020, 9, 13927–13936. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H.; Sheikh, F.A. Regenerated cellulose nanofibers from cellulose acetate: Incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111547. [Google Scholar] [CrossRef]
- Athukorala, S.S.; Liyanage, C.J.; Jayasundera, A.C.A. Hydroxyapatite incorporated bacterial cellulose hydrogels as a cost-effective 3D cell culture platform. Soft Mater. 2022, 20, 183–192. [Google Scholar] [CrossRef]
- Basu, P.; Saha, N.; Saha, P. Swelling and Rheological Study of Calcium Phosphate Filled Bacterial Cellulose-based Hydrogel Scaffold. J. Appl. Polym. Sci. 2020, 137, 48522. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, J.; Xiong, G.; Wan, Y. Evolution of Morphology of Bacterial Cellulose Scaffolds during Early Culture. Carbohydr. Polym. 2014, 111, 722–728. [Google Scholar] [CrossRef]
- Bayir, E.; Bilgi, E.; Hames, E.E.; Sendemir, A. Production of Hydroxyapatite–bacterial Cellulose Composite Scaffolds with Enhanced Pore Diameters for Bone Tissue Engineering Applications. Cellulose 2019, 26, 9803–9817. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Yang, L.; Chen, C.; Dou, R.; Yang, X.; Sun, B.; Zhou, B.; Zhang, L.; Sun, D. Enhanced mechanical properties and biocompatibility on BC/HAp composite through calcium gluconate fortified bacterial. Carbohydr. Polym. 2022, 281, 119085. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Chen, F.; Wei, Y.; Chen, X.; Zhou, Y.; Yang, X.; Zhu, X.; Tu, C.; Zhang, X. Nano-hydroxyapatite coating promotes porous calcium phosphate ceramic-induced osteogenesis via BMP/smad signaling pathway. Int. J. Nanomed. 2019, 14, 7987–8000. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-Y.; Won, J.-E.; Park, J.-S.; Lee, H.-H.; Kim, H.-W. Improvement of surface bioactivity of poly(lactic acid) biopolymer by sandblasting with hydroxyapatite bioceramic. Mater. Lett. 2011, 65, 2951–2955. [Google Scholar] [CrossRef]
- Hatano, K.; Inoue, H.; Kojo, T.; Matsunaga, T.; Tsujisawa, T.; Uchiyama, C.; Uchida, Y. Effect of surface roughness on proliferation and alkaline phosphatase expression of rat calvarial cells cultured on polystyrene. Bone 1999, 25, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Russias, J.; Saiz, E.; Nalla, R.K.; Gryn, K.; Ritchie, R.O.; Tomsia, A.P. Fabrication and me- chanical properties of PLA/HA composites: A study of in vitro degradation. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2006, 26, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Salamanca, E.; Tsao, T.-C.; Hseuh, H.-W.; Wu, Y.-F.; Choy, C.-S.; Lin, C.-K.; Pan, Y.-H.; Teng, N.-C.; Huang, M.-C.; Lin, S.-M.; et al. Fabrication of Polylactic Acid/β-Tricalcium Phosphate FDM 3D Printing Fiber to Enhance Osteoblastic-Like Cell Performance. Front. Mater 2021, 8, 683706. [Google Scholar] [CrossRef]
- Alksne, M.; Kalvaityte, M.; Simoliunas, E.; Rinkunaite, I.; Gendviliene, I.; Locs, J.; Rutkunas, V.; Bukelskiene, V. In vitro comparison of 3D printed polylactic acid/hydroxyapatite and polylactic acid/bioglass composite scaffolds: Insights into materials for bone regeneration. J. Mech. Behav. Biomed. Mater. 2020, 104, 103641. [Google Scholar] [CrossRef]
- Birgani, T.Z.; van Blitterswijk, C.A.; Habibovic, P. Monolithic calcium phosphate/poly(lactic acid) composite versus calcium phosphate-coated poly(lactic acid) for support of osteogenic differentiation of human mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2016, 27, 54. [Google Scholar] [CrossRef]
- Nevado, P.; Lopera, A.; Bezzon, V.; Fulla, M.R.; Palacio, J.; Zaghete, M.A.; Biasotto, G.; Montoya, A.; Rivera, J.; Robledo, S.; et al. Preparation and in vitro evaluation of PLA/biphasic calcium phosphate filaments used for fused deposition modelling of scaffolds. Mater. Sci. Eng. C 2020, 114, 111013. [Google Scholar] [CrossRef]
- Sahu, G.; Rajput, M.S.; Mahapatra, S.P. Polylactic acid nanocomposites for biomedical applications: Effects of calcium phosphate, and magnesium phosphate nanoparticles concentration. Plast. Rubber Comp. 2021, 5, 228–240. [Google Scholar] [CrossRef]
- Pérez, C.J.; Eisenberg, P.; Bernal, C.; Pérez, E. Mechanical evaluation of polylactic acid (PLA) based composites reinforced with different calcium phosphates. Mater. Res. Express 2018, 5, 105304. [Google Scholar] [CrossRef]
- Pérez, J.E. Mechanical performance of in vitro degraded polylactic acid/hydroxyapatite composites. J. Mater. Sci. 2021, 56, 19915–19935. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Alam, A.K.M.M.; Heim, H.P.; Feldmann, M. Synergized poly(lactic acid)–hydroxyapatite composites: Biocompatibility study Wiley Periodicals. Inc. J. Appl. Polym. Sci. 2019, 136, 47400. [Google Scholar] [CrossRef]
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Hardianti, D.; Hidayat, N.; Kurniawan, R. Study of Nano-Hydroxyapatite: Poly Lactide Acid (n-HA:PLA) Composites and Their Biocompatibility, Bioactivity, and Biodegradability Characteristics. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012034. [Google Scholar]
- Carvalho, T.S.S.; Ribeiro, N.; Torres, P.M.C.; Almeida, J.C.; Belo, J.H.; Araújo, J.P.; Ramos, A.; Oliveira, M.; Olhero, S.M. Magnetic polylactic acid-calcium phosphate-based biocomposite as a potential biomaterial for tissue engineering applications. Mater. Chem. Phys. 2023, 296, 127175. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, J.; Qin, C.; Xu, A.; Zhang, Z.; Lin, S.; Ren, X.; Zhang, P. Spin-coating synthesis, and characterization of Zn-doped hydroxyapatite/polylactic acid composite coatings. Surf. Coat. Technol. 2016, 307, 461–469. [Google Scholar] [CrossRef]
- Leonés, A.; Salaris, V.; Mujica-Garcia, A.; Arrieta, M.P.; Lopez, D.; Lieblich, M.; Kenny, J.M.; Peponi, L. PLA Electrospun Fibers Reinforced with Organic and Inorganic Nanoparticles: A Comparative Study. Molecules 2021, 26, 4925. [Google Scholar] [CrossRef]
- Tanaka, K.; Shiga, T.; Katayama, T. Fabrication of hydroxyapatite/PLA composite nanofiber by electrospinning. WIT Trans. Built Environ. 2016, 166, 372–379. [Google Scholar]
- Lopresti, F.; Pavia, F.C.; Vitrano, I.; Kersaudy-Kerhoas, M.; Brucato, V.; La Carrubba, V. Effect of hydroxyapatite concentration and size on morpho-mechanical properties of PLA-based randomly oriented and aligned electrospun nanofibrous mats. J. Mech. Behav. Biomed. Mater. 2020, 101, 103449. [Google Scholar] [CrossRef]
- Sóti, P.L.; Nagy, Z.K.; Serneels, G.; Vajna, B.; Farkas, A.; Gucht, F.V.D.; Fekete, P.; Vigh, T.; Wagner, I.; Balogh, A.; et al. Preparation and comparison of spray dried and electrospun bioresorbable drug delivery systems. Eur. Polym. J. 2015, 68, 671–679. [Google Scholar]
- Kim, G.M.; Asran, A.S.; Michler, G.H.; Simon, P.; Kim, J.S. Electrospun PVA/HAp nanocomposite nanofibers: Biomimetics of mineralized hard tissues at a lower level of complexity. Bioinspir. Biomim. 2008, 3, 046003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomater 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhi, Y.; Fang, H.; Wu, Z.; Chen, T.; Jiang, J.; Chen, S. Effects of polyvinylpyrrolidone-iodine on tendon-bone healing in a rabbit extra-articular model. Exp. Ther. Med. 2017, 13, 2751–2756. [Google Scholar] [CrossRef]
- Dau, M.; Ganz, C.; Zaage, F.; Frerich, B.; Gerber, T. Hydrogel-embedded nanocrystalline hydroxyapatite granules (elastic blocks) based on a cross-linked polyvinylpyrrolidone as bone grafting substitute in a rat tibia model. Int. J. Nanomed. 2017, 12, 7393–7404. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Seo, Y.H.; Oh, T.H. PVP Assisted Synthesis of Hydroxyapatite Nanorods with Tunable Aspect Ratio and Bioactivity. Hindawi Publishing Corporation. J. Nanomater. 2015, 2015, 621785. [Google Scholar] [CrossRef]
- Mukhanova, E.A.; Suprunova, I.A.; Suprunova, Y.A.; Zabiyaka, I.Y. Effect of the molecular weight of polyvinylpyrrolidone on the structure and morphology of materials based on substituted hydroxyapatite for bone implants. MATEC Web Conf. 2018, 226, 03012. [Google Scholar] [CrossRef]
- Ananth, K.P.; Guo, B.; Zhang, C.; Wang, W.; Zhou, P.; Bai, J. Investigation of biphasic calcium phosphate (BCp)/polyvinylpyrrolidone (PVp)/graphene oxide (GO) composite for biomedical implants. Ceram. Int. 2020, 46, 24413–24423. [Google Scholar] [CrossRef]
- Guesmi, Y.; Agougui, H.; Jabli, M.; Alsharabasy, A.M. Bioactive composites of hydroxyapatite/polyvinylpyrrolidone for bone regeneration applications. Chem. Eng. Commun. 2018, 206, 279–288. [Google Scholar] [CrossRef]
- Mathina, M.; Shinyjoy, E.; Kavitha, L.; Gopi, D. Biowaste-derived hydroxyapatite reinforced with polyvinyl pyrrolidone/aloevera composite for biomedical applications. Appl. Ceram. Technol. 2021, 18, 221–234. [Google Scholar] [CrossRef]
- McKeen, L. Chapter11—The effect of heat aging on the properties of sustainable polymers. In The Effect of Long Term Thermal Exposure on Plastics and Elastomers, 2nd ed.; William Andrew: Norwich, NY, New York, 2021; pp. 313–332. [Google Scholar]
- Juan, P.-K.; Fan, F.-Y.; Lin, W.-C.; Liao, P.-B.; Huang, C.-F.; Shen, Y.-K.; Ruslin, M.; Lee, C.-H. Bioactivity and Bone Cell Formation with Poly-ε-Caprolactone/Bioceramic 3D Porous Scaffolds. Polymers 2021, 13, 2718. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ye, X.J.; Wei, D.X.; Zhong, J.; Chen, Y.; Xu, G.; He, D. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair. ACS Appl. Mater. Interfaces 2014, 6, 14952–14963. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Antunović, M.; Gallego-Ferrer, G.; Ivanković, M.; Ivanković, H. PCL-Coated Multi-Substituted Calcium Phosphate Bone Scaffolds with Enhanced Properties. Materials 2021, 14, 4403. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, N.; Arefian, E.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. 3D-Printed PCL Scaffolds Coated with Nanobioceramics Enhance Osteogenic Differentiation of Stem Cells. ACS Omega 2021, 6, 35284–35296. [Google Scholar] [CrossRef]
- Petit, C.; Tulliani, J.M.; Tadier, S.; Meille, S.; Chevalier, J.; Palmero, P. Novel calcium phosphate/PCL graded samples: Design and development in view of biomedical applications. Mater. Sci. Eng. C 2019, 97, 336–346. [Google Scholar] [CrossRef]
- Ressler, A.; Bauer, L.; Prebeg, T.; Ledinski, M.; Hussainova, I.; Urli´c, I.; Ivankovi´c, M.; Ivankovi´c, H. PCL/Si-Doped Multi-Phase Calcium Phosphate Scaffolds Derived from Cuttlefish Bone. Materials 2022, 15, 3348. [Google Scholar] [CrossRef]
- Chunyan, Z.; Lan, C.; Jiajia, L.; Dongwei, S.; Jun, Z.; Huinan, L. In vitro evaluation of degradation, cytocompatibility and antibacterial property of polycaprolactone/hydroxyapatite composite coating on bioresorbable magnesium alloy. J. Magnes. Alloy. 2022, 10, 2252–2265. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, S.; Iqbal, T.; Bakhsheshi-rad, H.R.; Alsakkak, A.; Kamil, A.; KadirADIR, M.R.A.; Idris, M.H.; Raghav, H.B. Zinc-doped hydroxyapatite−zeolite/polycaprolactone composites coating on magnesium substrate for enhancing in-vitro corrosion and antibacterial performance. Trans. Nonferrous Met. Soc. China 2020, 30, 123–133. [Google Scholar] [CrossRef]
- Sing, N.; Batra, U.; Kumar, K.; Mahapatro, A. Investigating TiO2–HA–PCL hybrid coating as an efficient corrosion resistant barrier of ZM21 Mg alloy. J. Magnes. Alloy. 2021, 9, 627–646. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Zhou, J.; Li, S.-Y.; Huang, P.-R. In vitro corrosion resistance and cytocompatibility of Mg66Zn28Ca6 amorphous alloy materials coated with a double-layered nHA and PCL/nHA coating. Colloids Surf. B Biointerfaces 2020, 196, 111251. [Google Scholar] [CrossRef]
- Ansari, Z.; Kalantar, M.; Kharaziha, M.; Ambrosio, L.; Raucci, M.G. Polycaprolactone/fluoride substituted-hydroxyapatite (PCL/FHA) nanocomposite coatings prepared by in-situ sol-gel process for dental implant applications. Prog. Org. Coat. 2020, 147, 105873. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Jin, X.; Ke, Q. An electrospun poly(3-caprolactone) nanocomposite fibrous mat with a high content of hydroxyapatite to promote cell infiltration. RSC Adv. 2018, 8, 25228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linh, N.V.V.; Du, N.T.; My, N.T.N.; Tuyen, N.N.; Phu, H.D.; Tram, N.X.T. Electrospun Polycaprolactone/Hydroxyapatite (PCL/HAp) microfibers as potential biomaterials for tissue engineering. Mater. Today Proc. 2022, 66, 2895–2899. [Google Scholar] [CrossRef]
- Montañez, N.D.; Carreño, H.; Escobar, P.; Estupiñán, H.A.; Peña, D.Y.; Goel, S.; Endrino, J.L. Functional evaluation and testing of a newly developed Teleost’s Fish Otolith derived biocomposite coating for healthcare. Sci. Rep. 2020, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vu, A.A.; Emshadi, K.; Bandyopadhyay, A. Effects of polycaprolactone on alendronate drug release from Mg-doped hydroxyapatite coating on titanium. Mater. Sci. Eng. C 2018, 88, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Iynoon Jariya, S.A.; Babu, A.A.; Sankara Narayanan, T.S.N.; Vellaichamy, E.; Ravichandran, K. Development of a novel smart carrier for drug delivery: Ciprofloxacin loaded vaterite/reduced graphene oxide/PCL composite coating on TiO2 nanotube coated titanium. Ceram. Int. 2022, 48, 9579–9594. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Patil, S.B.; Inamdar, S.Z.; Reddy, K.R.; Raghu, A.V.; Soni, S.K.; Kulkarni, R.V. Novel biocompatible poly(acrylamide)-grafteddextran hydrogels: Synthesis, characterization and biomedical applications. J. Microbiol. Methods 2019, 159, 200–210. [Google Scholar] [CrossRef]
- Nasibi, S.; Khoramabadi, H.N.; Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxiy methyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 68–75. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Tiwari, S.; Dharmalingam, K.; Shukla, I.; Pandey, L.M. Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J. Biomed. Mater. Res. Part A 2017, 105, 2391–2404. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, V. Crosslinking of poly(vinylpyrrolidone)/acrylic acid with tragacanth gum for hydrogels formation for use in drug delivery applications. Carbohydr. Polym. 2017, 157, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, K.; Lytkina, D.; Sadykov, R.; Shalygina, K.; Khojazoda, T.; Mahmadbegov, R.; Kurzina, I. Composite Cement Materials Based on β-Tricalcium Phosphate, Calcium Sulfate, and a Mixture of Polyvinyl Alcohol and Polyvinylpyrrolidone Intended for Osteanagenesis. Polymers 2023, 15, 210. [Google Scholar] [CrossRef]
- Buchsel, P.C.; Murphy, P.J.M. Polyvinylpyrrolidone–sodium hyaluronate gel (Gelclair®): A bioadherent oral gel for the treatment of oral mucositis and other painful oral lesions. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Trofimchuk, E.S.; Forysenkova, A.A.; Ahmed, A.I.; Gnezdilov, O.I.; Davydova, G.A.; Kozlova, S.G.; Antoniac, A.; Rau, J.V. Composite Polyvinylpyrrolidone–Sodium Alginate—HydroxyapatiteHydrogel Films for Bone Repair and Wound Dressings Applications. Polymers 2021, 13, 3989. [Google Scholar] [CrossRef]
- Kandasamy, S.; Narayanan, V.; Sumathi, S. Zinc and manganese substituted hydroxyapatite/CMC/PVP electrospun composite, for bone repair applications. Int. J. Biol. Macromol. 2020, 145, 1018–1030. [Google Scholar] [CrossRef]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic bone scaffold design parameters: Osteogenic differentiation and signal expression. Tissue Eng. Part B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef]
- Wang, T.; Yang, X.; Qi, X.; Jiang, C. Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (ε-caprolactone)/hydroxyapatite/collagen scaffolds. J. Trans. Med. 2015, 13, 152. [Google Scholar] [CrossRef]
- Linh, N.T.B.; Min, Y.K.; Lee, B.T. Hybrid hydroxyapatite nanoparticles-loaded PCL/GE blend fibers for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 5, 520–538. [Google Scholar] [CrossRef]
- Kichi, M.K.; Torkaman, R.; Mohammadi, H.; Toutounchi, A.; Kharaziha, M.; Alihosseini, F. Electrochemical and in vitro bioactivity behavior of Poly(caprolactone)(PCL)-Gelatin-Forsterite NanoCoating on Titanium for Biomedical Application. Mater. Today Commun. 2020, 24, 101326. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Enhanced osteogenic differentiation of stem cells by 3D printed PCL scaffolds coated with collagen and hydroxyapatite. Sci. Rep. 2022, 12, 12359. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tong, H.; Shen, X.; Chen, W.; Yan, J.; Hu, J. Preparation and characterization of homogeneous chitosan–polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 2009, 5, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically Biomimetic Bone Scaffold Materials: Nano-HA/Collagen/PLA Composite. J. Biomed. Mater. Res. B 2004, 69, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Shahruzzaman, M.; Islam, M.S.; Khan, M.N.; Haque, P. Preparation and properties of biodegradable polymer/nano-hydroxyapatite bioceramic scaffold for spongy bone regeneration. J. Polym. Eng. 2019, 39, 134–142. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenhof, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocyclinenanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J. Tissue Eng. Regen. Med. 2013, 7, 85. [Google Scholar] [CrossRef]
- Ma, R.; Xiong, D.; Miao, F.; Zhang, J.; Peng, Y. Novel PVP/PVA hydrogels for articular cartilage replacement. Mater. Sci. Eng. C 2009, 29, 1979. [Google Scholar] [CrossRef]
- Katta, J.K.; Marcolongo, M.; Lowman, A.; Mansmann, K.A. Friction and wear behavior of poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for articular cartilage replacement. J. Biomed. Mater. Res. A 2007, 83, 471–479. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, D. Microstructure and friction properties of PVA/PVP hydrogels for articular cartilage repair as function of polymerization degree and polymer concentration. Wear 2013, 305, 280. [Google Scholar] [CrossRef]
- Zidan, H.M.; Abdelrazek, E.M.; Abdelghany, A.M.; Tarabiah, A.E. Characterization and some physical studies of PVA/PVP filled with MWCNTs. J. Mater. Res. Technol. 2019, 8, 904–913. [Google Scholar] [CrossRef]
- Rahmani, F.; Ziyadi, H.; Baghali, M.; Luo, H.; Ramakrishna, S. Electrospun PVP/PVA Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management. Appl. Sci. 2021, 11, 2779. [Google Scholar] [CrossRef]
- Sedlarik, V.; Saha, N.; Kuritka, I.; Saha, P. Characterization of polymeric biocomposite based on poly(vinyl alcohol) and poly(vinyl pyrrolidone. Polym. Compos. 2006, 27, 147. [Google Scholar] [CrossRef]
- Abou Taleb, M.H. Thermal and spectroscopic studies of poly(N-vinyl pyrrolidone)/poly(vinyl alcohol) blend films. J. Appl. Polym. Sci. 2009, 114, 1202. [Google Scholar] [CrossRef]
- Maheshwari, S.U.; Govindan, K.; Raja, M.; Raja, A.; Pravin, M.B.S.; Kumar, S.V. Preliminary studies of PVA/PVP blends incorporated with HAp and β-TCP bone ceramic as template for hard tissue engineering. Bio-Med. Mater. Eng. 2017, 28, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bai, T.; Wang, F. The physical and chemical properties of the polyvinylalcohol/polyvinylpyrrolidone/hydroxyapatite composite hydrogel. Mater. Sci. Eng. C 2016, 59, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Lowman, A.; Marcolongo, M. Novel associated hydrogels for nucleus pulposus replacement. J. Biomed. Mater. Res. A 2003, 67, 1329–1337. [Google Scholar] [CrossRef]
- Kanca, Y.; Milner, P.; Dini, D.; Amis, A.A. Tribological properties of PVA/PVP blend hydrogels against articular cartilage. J. Mech. Behav. Biomed. Mater. 2018, 78, 36–45. [Google Scholar] [CrossRef]
- Ma, R.; Xiong, D.; Miao, F.; Zhang, J.; Peng, Y. Friction properties of novel PVP/PVA blend hydrogels as artificial cartilage. J. Biomed. Mater. Res. A 2010, 93, 1016–1019. [Google Scholar] [CrossRef]
- Åkerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D-Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef]
- Etminanfar, M.R.; Sheykholeslami, S.O.R.; Khalili, V.; Mahdavi, S. Biocompatibility and drug delivery efficiency of PEG-b-PCL/hydroxyapatite bilayer coatings on nitinol superelastic alloy. Ceram. Int. 2020, 46, 12711–12717. [Google Scholar] [CrossRef]
- Mystiridou, E.; Patsidis, A.C.; Bouropoulos, N. Development and Characterization of 3D Printed Multifunctional Bioscaffolds Based on PLA/PCL/HAp/BaTiO3 Composites. Appl. Sci. 2021, 11, 4253. [Google Scholar] [CrossRef]
- Abbas, Z.; Dapporto, M.; Tampieri, A.; Sprio, S. Toughening of Bioceramic Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 259. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing Risk of Failure from Ceramic-on-Ceramic Total Hip Prosthesis by Selecting Ceramic Materials Based on Tresca Stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]



| Polymer Matrices | Properties | Form | Applications |
|---|---|---|---|
| Collagen | Biodegradable, biocompatible, cytocompatible, bioactive, low mechanical properties | Hydrogels, 3D scaffolds, film coatings | Bone grafts, bone and tissue engineering, pharmaceutical |
| Gelatin | Biodegradable, biocompatible, non-immunogenic, bioactive, injectable, low mechanical properties | Hydrogels, 3D scaffolds, film coatings | Bone-to-implant bonding, bone grafts, tissue engineering, cartilage, pharmaceutical |
| Chitosan | Biodegradable, biocompatible, water-soluble, slightly antibacterial and antioxidant, low mechanical properties | Hydrogels 3D scaffolds, membranes, film coatings | Bone and tissue engineering, energy and environmental applications, food packaging, pharmaceutical, drug carrier |
| Alginate | Biocompatible, water-soluble, high viscosity, suspending agent, film-forming ability | Hydrogels 3D scaffolds, membranes, film coatings | Bone and tissue engineering, prosthesis, dental molds, and impression materials, pharmaceutical, drug carrier |
| Cellulose | Biocompatible, bioactive, biodegradable, non-water-soluble, high water permeability, film-forming ability, mechanical strength, osteoconductivity | Hydrogels 3D scaffolds, membranes, film coatings | Bone and tissue engineering, drug delivery, bone graft, drug carrier |
| Polymer Matrices | Properties | Form | Applications |
|---|---|---|---|
| Polylactic acid (PLA) | Biodegradable, biocompatible, good mechanical properties, non-water soluble, hydrophilic, slow biodegradation rate | 3D-printed scaffolds, films, fibers. | Bone grafts, bone and tissue engineering, pharmaceutical, regenerative medicine, drug carriers, dental material, coatings on orthopedic implants |
| Polyvinylpyrrolidone (PVP) | Biodegradable, biocompatible, highly water soluble, low mechanical properties | hydrogel, fibers | Surfactant, filling agent in tissue engineering, bone graft, cartilage, pharmaceutical |
| Polycaprolactone (PCL) | Biodegradable, biocompatible, non-water soluble, hydrophobic, good mechanical properties, implantable | 3D-printed scaffolds, membranes, films, fibers | Bone and tissue engineering, drug carriers, drug delivery, dental material, coatings on orthopedic implants, pharmaceutical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furko, M.; Balázsi, K.; Balázsi, C. Calcium Phosphate Loaded Biopolymer Composites—A Comprehensive Review on the Most Recent Progress and Promising Trends. Coatings 2023, 13, 360. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13020360
Furko M, Balázsi K, Balázsi C. Calcium Phosphate Loaded Biopolymer Composites—A Comprehensive Review on the Most Recent Progress and Promising Trends. Coatings. 2023; 13(2):360. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13020360
Chicago/Turabian StyleFurko, Monika, Katalin Balázsi, and Csaba Balázsi. 2023. "Calcium Phosphate Loaded Biopolymer Composites—A Comprehensive Review on the Most Recent Progress and Promising Trends" Coatings 13, no. 2: 360. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13020360