Chemical and Biological Roles of Zinc in a Porous Titanium Dioxide Layer Formed by Micro-Arc Oxidation
Abstract
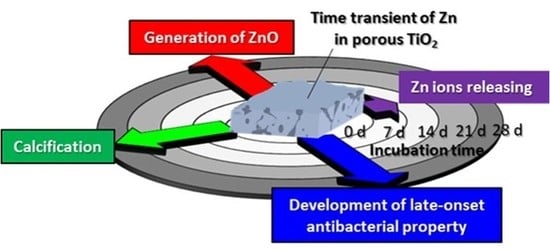
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Incubation in Saline
2.3. Surface Characterization and Evaluation of Zinc Ion Release
2.4. Evaluation of Antibacterial Activity
2.5. Calcification by Osteogenic Cells
2.6. Statistical Analysis
3. Results
3.1. Surface Characterization and Evaluation of Zinc Ion Release
3.2. Evaluation of Antibacterial Activity
3.3. Calcification by Osteogenic Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grainger, D.W.; van der Mei, H.C.; Jutte, P.C.; van den Dungen, J.J.A.M.; Schultz, M.J.; van der Laan, B.F.A.M.; Zaat, S.A.J.; Busscher, H.J. Critical factors in the translation of improved antimicrobial strategies for medical implants and devices. Biomaterials 2013, 34, 9237–9243. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Dibart, S.; Warbington, M.; Su, M.F.; Skobe, Z. In vitro evaluation of the implant-abutment bacterial seal: The locking taper system. Int. J. Oral Maxillofac. Implant. 2005, 20, 732–737. [Google Scholar]
- Glauser, R.; Schupbach, P.; Gottlow, J.; Hammerle, C.H. Periimplant soft tissue barrier at experimental one-piece mini-implants with different surface topography in humans: A light-microscopic overview and histometric analysis. Clin. Implant Dent. Relat. Res. 2005, 7, S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, M.; Wallet, S.; Koutouzis, T.; Lundgren, T. Bacterial colonization of the dental implant fixture-abutment interface: An in vitro study. J. Periodontol. 2009, 80, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, E.E.; Patel, J.D.; Marchant, R.E.; Anderson, J.M. Effects of biomaterial surface chemistry on the adhesion and biofilm formation of staphylococcus epidermidis in vitro. J. Biomed. Mater. Res. A 2006, 78A, 836–842. [Google Scholar] [CrossRef]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef]
- Lindsay, D.; von Holy, A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J. Hosp. Infect. 2006, 64, 313–325. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 34, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Conventry, M.B. Treatment of infections occurring in total hip surgery. Orthop. Clin. North Am. 1975, 6, 991–1003. [Google Scholar]
- LaPorte, D.M.; Waldman, B.J.; Mont, M.A.; Hungerford, D.S. Infections associated with dental procedures in total hip arthroplasty. J. Bone Joint Surg. Br. 1999, 81B, 56–59. [Google Scholar] [CrossRef]
- Rubin, R.; Salvati, E.A.; Lewis, R. Infected total hip-replacement after dental procedures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1976, 41, 18–23. [Google Scholar] [CrossRef]
- Koerner, R.J.; Butterworth, L.A.; Mayer, I.V.; Dasbach, R.; Busscher, H.J. Bacterial adhesion to titanium-oxy-nitride (TiNOX) coatings with different resistivities: A novel approach for the development of biomaterials. Biomaterials 2002, 23, 2835–2840. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.Q.; Huang, X.B.; Zhao, L.Z.; Zhang, X.Y.; Wang, L.; Tang, B.; Ma, S.L.; Chu, P.K. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Wang, H.R.; Huo, K.F.; Cui, L.Y.; Zhang, W.R.; Ni, H.W.; Zhang, Y.M.; Wu, Z.F.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Cao, H.L.; Liu, X.Y.; Meng, F.H.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Mei, S.L.; Wang, H.Y.; Wang, W.; Tong, L.P.; Pan, H.B.; Ruan, C.S.; Ma, Q.L.; Liu, M.Y.; Yang, H.L.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef]
- Gasqueres, C.; Schneider, G.; Nusko, R.; Maier, G.; Dingeldein, E.; Eliezer, A. Innovative antibacterial coating by anodic spark deposition. Surf. Coat. Technol. 2012, 206, 3410–3414. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Yamada, R.; Ashida, M.; Chen, P.; Doi, H.; Nozaki, K.; Nagai, A.; Hanawa, T. Investigation of realizing both antibacterial property and osteogenic cell compatibility on titanium surface by simple electrochemical treatment. ACS Biomater. Sci. Eng. 2019, in press. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nunez, N.V.; Turrent, L.D.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of mimusops elengi, linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. Engl. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Samani, S.; Hossainalipour, S.M.; Tamizifar, M.; Rezaie, H.R. In vitro antibacterial evaluation of sol–gel-derived Zn-, Ag-, and (Zn + Ag)-doped hydroxyapatite coatings against methicillin-resistant staphylococcus aureus. J. Biomed. Mater. Res. A 2013, 101, 222–230. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef] [PubMed]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-based anti-infectives: Targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.A.; Webster, T.J. Antimicrobial selenium nanoparticle coatings on polymeric medical devices. Nanotechnology 2013, 24, 155101. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Valencia, C.; Lopez-Alvarez, M.; Cochon-Cores, B.; Pereiro, I.; Serra, J.; Gonzalez, P. Novel selenium-doped hydroxyapatite coatings for biomedical applications. J. Biomed. Mater. Res. A 2013, 101, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Niu, J.F.; Chen, Y.S. Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects. Langmuir 2013, 29, 4647–4651. [Google Scholar] [CrossRef] [PubMed]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Storrie, H.; Stupp, S.I. Cellular response to zinc-containing organoapatite: An in vitro study of proliferation, alkaline phosphatase activity and biomineralization. Biomaterials 2005, 26, 5492–5499. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Cockerill, I.; Wang, Y.D.; Qin, Y.X.; Chang, L.Q.; Zheng, Y.F.; Zhu, D.H. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Titanium–tissue interface reaction and its control with surface treatment. Front Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. A comprehensive review of techniques for biofunctionalization of titanium. J. Periodontal. Implant. Sci. 2011, 41, 263–272. [Google Scholar] [CrossRef]
- Ha, J.Y.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Kim, K.H.; Hanawa, T. Enhancement of calcium phosphate formation on zirconium by micro-arc oxidation and chemical treatments. Surf. Coat. Technol. 2011, 205, 4948–4955. [Google Scholar] [CrossRef]
- Song, W.H.; Ryu, H.S.; Hong, S.H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coating formed by micro-arc oxidation. J. Biomed. Mater. Res. A 2009, 88A, 246–254. [Google Scholar] [CrossRef]
- Li, L.H.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef]
- Li, Y.; Lee, I.S.; Cui, F.Z.; Choi, S.H. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium. Biomaterials 2008, 29, 2025–2032. [Google Scholar] [CrossRef]
- Suh, J.Y.; Janga, B.C.; Zhu, X.; Ong, J.L.; Kim, K.H. Effect of hydrothermally treated anodic oxide films on osteoblast attachment and proliferation. Biomaterials 2003, 24, 347–355. [Google Scholar] [CrossRef]
- Son, W.W.; Zhu, X.; Shin, H.I.; Ong, J.L.; Kim, K.H. In vivo histological response to anodized and anodized/hydrothermally treated titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66B, 520–525. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.; Kim, H.E.; Koh, Y.H.; Kim, H.W.; Jang, J.H. Formation of hydroxyapatite within porous TiO2 layer by micro-arc oxidation coupled with electrophoretic deposition. Acta Biomater. 2009, 5, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.J.; Chen, C.Z.; Zhao, Z.H. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Wang, L.; Shi, L.; Chen, J.J.; Shi, Z.F.; Ren, L.; Wang, Y.J. Biocompatibility of Si-incorporated TiO2 film prepared by micro-arc oxidation. Mater. Lett. 2014, 116, 35–38. [Google Scholar] [CrossRef]
- Zhang, R.F.; Qiao, L.P.; Qu, B.; Zhang, S.F.; Chang, W.H.; Xiang, J.H. Biocompatibility of micro-arc oxidation coatings developed on Ti6Al4V alloy in a solution containing organic phosphate. Mater. Lett. 2015, 153, 77–80. [Google Scholar] [CrossRef]
- Chen, H.T.; Chung, C.J.; Yang, T.C.; Chiang, I.P.; Tang, C.H.; Chen, K.C.; He, J.L. Osteoblast growth behavior on micro-arc oxidized β-titanium alloy. Surf. Coat. Technol. 2010, 205, 1624–1629. [Google Scholar] [CrossRef]
- Cimenoglu, H.; Gunyuz, M.; Kose, G.T.; Baydogan, M.; Ugurlu, F.; Sener, C. Micro-arc oxidation of Ti6Al4V and Ti6Al7Nb alloys for biomedical applications. Mater. Charact. 2011, 62, 304–311. [Google Scholar] [CrossRef]
- The in vitro and in vivo performance of a strontium-containing coating on the low-modulus Ti35Nb2Ta3Zr alloy formed by micro-arc oxidation. J. Mater. Sci. Mater. Med. 2015, 26, 203. [CrossRef]
- He, X.J.; Zhang, X.Y.; Wang, X.; Qin, L. Review of Antibacterial activity of titanium based implants’ surface fabricated by micro-arc oxidation. Coatings 2017, 7, 45. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta. Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef]
- Zhao, B.H.; Zhang, W.; Wang, D.N.; Feng, W.; Liu, Y.; Lin, Z.; Du, K.Q.; Deng, C.F. Effect of Zn content on cytoactivity and bacteriostasis of micro-arc oxidation coatings on pure titanium. Surf. Coat. Technol. 2013, 228, S428–S432. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Q.; Han, Y. Zn and Ag co-doped anti-microbial TiO2 coatings on Ti by micro-arc oxidation. J. Mater. Sci. Technol. 2016, 32, 919–924. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, H.Z.; Li, J.F.; He, X.J.; Hang, R.Q.; Huang, X.B.; Tian, L.H.; Tang, B. Corrosion behavior of Zn-incorporated antibacterial TiO2 porous coating on titanium. Ceram. Int. 2016, 42, 17095–17100. [Google Scholar] [CrossRef]
- Du, Q.; Wei, D.Q.; Liu, S.; Cheng, S.; Hu, N.; Wang, Y.M.; Li, B.Q.; Jia, D.C.; Zhou, Y. The hydrothermal treated Zn-incorporated titania based microarc oxidation coating: Surface characteristics, apatite-inducing ability and antibacterial ability. Surf. Coat. Technol. 2013, 352, 489–500. [Google Scholar] [CrossRef]
- Zhang, X.X.; Yang, L.; Lu, X.Q.; Lv, Y.; Jiang, D.; Yu, Y.; Peng, Z.; Dong, Z.H. Characterization and property of dual-functional Zn-incorporated TiO2 micro-arc oxidation coatings: The influence of current density. J. Alloy. Compd. 2019, 810, 151893. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kobayashi, E.; Hiromoto, S.; Asami, K.; Imai, H.; Hanawa, T. Calcium phosphate formation on Ti by low-voltage electrolytic treatments. J. Mater. Sci. Mater. Med. 2007, 18, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 552–556. [Google Scholar] [CrossRef]
- Asami, K.; Hashimoto, K.; Shimodaira, S. XPS determination of compositions of alloy surfaces and surface oxides on mechanically polished iron–chromium alloys. Corros. Sci. 1977, 17, 713–723. [Google Scholar] [CrossRef]
- Asami, K.; Chen, S.C.; Habazaki, H.; Kawashima, A.; Hashimoto, K. A photoelectrochemical and ESCA study of passivity of amorphous nickel-valve metal alloys. Corros. Sci. 1990, 31, 727–732. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kasaya, M.; Asami, K.; Masumoto, T. Electrochemical and XPS studies on corrosion behavior of amorphous Ni–Cr–P–B alloys. Corros. Eng. 1977, 26, 445–452. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Oya, K.; Tanaka, Y.; Moriyama, Y.; Yoshioka, Y.; Kimura, T.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Noda, K.; Kishida, A.; et al. Differences in the bone differentiation properties of MC3T3-E1 cells on polished bulk and sputter-deposited titanium specimens. J. Biomed. Mater. Res. A 2010, 94A, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Moretti, G.; Fierro, G.; Jacono, M.L.; Porta, P. Characterization of CuO–ZnO catalysts by X-ray photoelectron spectroscopy: Precursors, calcined and reduced samples. Surf. Interface. Anal. 1989, 14, 325–336. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Gaarenstroom, S.W.; Winograd, N. Initial and final state effects in the ESCA spectra of cadmium and silver oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Du, W.L.; Niu, S.S.; Xu, Y.L.; Xu, Z.R.; Fan, C.L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the mechanism of antibacterial activity of ZnO: Surface defects mediated reactive oxygen species even in the dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- McWhirter, M.J.; Bremer, P.J.; Lamont, I.L.; McQuillan, A.J. Siderophore-Mediated covalent bonding to metal (Oxide) surfaces during biofilm initiation by pseudomonas aeruginosa bacteria. Langmuir 2003, 19, 3575–3577. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, R.K.; Gaur, K.; Torres, J.F.C.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a hot debate on the application of TiO2 nanoparticles in Sunscreen. Materials 2019, 12, 2317. [Google Scholar] [CrossRef]
- Jia, Z.J.; Xiu, P.; Li, M.; Xu, X.C.; Shi, Y.Y.; Cheng, Y.; Wei, S.C.; Zheng, Y.F.; Xi, T.F.; Cai, H.; et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef]
- Tsutsumia, Y.; Niinomi, M.; Nakai, M.; Tsutsumi, H.; Doi, H.; Nomura, N.; Hanawa, T. Micro-arc oxidation treatment to improve the hard-tissue compatibility of Ti–29Nb–13Ta–4.6Zr alloy. Appl. Surf. Sci. 2012, 262, 34–38. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. J. Biomed. Mater. Res. 1998, 39, 331–340. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimabukuro, M.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Doi, H.; Nagai, A.; Hanawa, T. Chemical and Biological Roles of Zinc in a Porous Titanium Dioxide Layer Formed by Micro-Arc Oxidation. Coatings 2019, 9, 705. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9110705
Shimabukuro M, Tsutsumi Y, Nozaki K, Chen P, Yamada R, Ashida M, Doi H, Nagai A, Hanawa T. Chemical and Biological Roles of Zinc in a Porous Titanium Dioxide Layer Formed by Micro-Arc Oxidation. Coatings. 2019; 9(11):705. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9110705
Chicago/Turabian StyleShimabukuro, Masaya, Yusuke Tsutsumi, Kosuke Nozaki, Peng Chen, Risa Yamada, Maki Ashida, Hisashi Doi, Akiko Nagai, and Takao Hanawa. 2019. "Chemical and Biological Roles of Zinc in a Porous Titanium Dioxide Layer Formed by Micro-Arc Oxidation" Coatings 9, no. 11: 705. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9110705