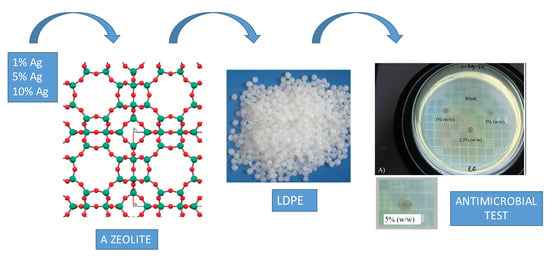
Synthesis of Antimicrobial Films Based on Low-Density Polyethylene (LDPE) and Zeolite A Containing Silver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silver Incorporation in Zeolite A
2.3. Calculation of Crystallinity of Zeolite A
2.4. Preparation of Composite Films
2.5. Characterization of Materials
2.6. Antimicrobial Activity of Silver Exchanged Zeolite A
2.7. Antimicrobial Activity of Composite Films
3. Results and Discussion
3.1. Materials Characterization
3.2. Minimum Inhibitory Concentration of Zeolite Impregnated with Different Silver Contents
3.3. Antimicrobial Activity of the Polymer Containing Zeolite Impregnated with Silver
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Osés, J.; Fuentes, G.G.; Palacio, J.F.; Esparza, J.; García, J.A.; Rodríguez, R. Antibacterial Functionalization of PVD Coatings on Ceramics. Coatings 2018, 8, 197. [Google Scholar] [CrossRef]
- Jiraroj, D.; Tungasmita, S.; Tungasmita, D.N. Silver ions and silver nanoparticles in zeolite a composites for antibacterial activity. Powder Technol. 2014, 264, 418–422. [Google Scholar] [CrossRef]
- Pehlivan, H.; Balkose, D.; Ulku, S.; Tihminlioglu, F. Characterization of pure and silver exchanged natural zeolite filled polypropylene composite films. Compos. Sci. Technol. 2005, 65, 2049–2058. [Google Scholar] [CrossRef]
- Zhao, G.; Stevens, S.E. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. BioMetals 1998, 11, 27–32. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal Actions of a Silver Ion Solution on Escherichia coli, Studied by Energy-Filtering Transmission Electron Microscopy and Proteomic Analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef]
- Tosheva, L.; Belkhair, S.; Gackowski, M.; Malic, S.; Al-Shanti, N.; Verran, J. Rapid screening of the antimicrobial efficacy of Ag zeolites. Colloids Surf. B 2017, 157, 254–260. [Google Scholar] [CrossRef]
- Cerrillo, J.L.; Palomares, A.E.; Rey, F.; Valencia, S.; Palou, L.; Pérez-Gago, M.B. Ag-zeolites as fungicidal material: Controlo f citrus green mold caused by Penicillium digitatum. Microporous Mesoporous Mater. 2017, 254, 69–76. [Google Scholar] [CrossRef]
- Ferreira, L.; Fonseca, A.M.; Botelho, G.; Almeida-Aguiar, C.; Neves, I.C. Antimicrobial activity of faujasite zeolites doped with silver. Microporous Mesoporous Mater. 2012, 160, 126–132. [Google Scholar] [CrossRef]
- Roy, A.; Butola, B.S.; Joshi, M. Synthesis, characterization and antibacterial properties of novel nano-silver loaded acid activated montmorillonite. Appl. Clay Sci. 2017, 146, 278–285. [Google Scholar] [CrossRef]
- Malachová, K.; Praus, P.; Rybková, Z.; Kozák, O. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl. Clay Sci. 2011, 53, 642–645. [Google Scholar] [CrossRef]
- Cruz-Pacheco, A.F.; Muñoz-Castiblanco, D.T.; Cuaspud, J.A.G.; Paredes-Madrid, L.; Vargas, C.A.P.; Zambrano, J.J.M.; Gómez, C.A.P. Coating of Polyetheretherketone Films with Silver Nanoparticles by a Simple Chemical Reduction Method and Their Antibacterial Activity. Coatings 2019, 9, 91. [Google Scholar] [CrossRef]
- Boschetto, D.L.; Lerin, L.; Cansian, R.; Pergher, S.B.C.; Di Luccio, M. Preparation and antimicrobial activity of polyethylene composite films with silver exchanged zeolite-Y. Chem. Eng. J. 2012, 204, 210–216. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Sanches-Silva, A.; Motta, J.F.G.; Andrade, M.; Neves, I.A.; Teófilo, R.F.; Carvalho, M.G.; Melo, N.R. Combined use of essential oils applied to protein base active food packaging: Study in vitro and in a food simulant. Eur. Polym. J. 2017, 93, 75–86. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Efficacy of polyethylene-based antimicrobial films containing principal constituents of basil. LWT-Food Sci. Technol. 2008, 41, 779–788. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Salmiéri, S.; Saucier, L.; Lacroix, M. Antimicrobial and Antioxidant Effects of Milk Protein-Based Film Containing Essential Oils for the Preservation of Whole Beef Muscle. J. Agric. Food Chem. 2004, 52, 5598–5605. [Google Scholar] [CrossRef]
- Mecitoglu, Ç.; Yemenicioglu, A.; Arslanoglu, A.; Elmaci, Z.S.; Korel, F.; Çetin, A.E. Incorporation of partially purified hen egg whitelysozyme into zein films for antimicrobial food packaging. Food Res. Int. 2006, 39, 12–21. [Google Scholar] [CrossRef]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. High-pressure processing and antimicrobial biodegradable packaging to control Listeria monocytogenes during storage of cooked ham. Food Microbiol. 2008, 25, 177–182. [Google Scholar] [CrossRef]
- Kamisoglu, K.; Aksoy, E.A.; Akata, B.; Hasirci, N.; Baç, N. Preparation and Characterization of Antibacterial Zeolite-Polyurethane Composites. J. Appl. Polym. Sci. 2008, 110, 2854–2861. [Google Scholar] [CrossRef]
- Fernández, A.; Soriano, E.; Hernández-Muñoz, P.; Gavara, R. Migration of antimicrobial silver from composites of polylactide with silver zeolites. J. Food Sci. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Zampino, D.; Ferreri, T.; Puglisi, C.; Mancuso, M.; Zaccone, R.; Scaffaro, R.; Bennardo, D. PVC silver zeolite composites with antimicrobial properties. J. Mater. Sci. 2011, 46, 6734–6743. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- International Zeolite Association. 2019. Available online: http://iza-online.org/synthesis/default.htm (accessed on 9 November 2019).
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- NCCLS. Performance Standards for Antimicrobial Disk Susceptibility Tests; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2003. [Google Scholar]
- Fonseca, A.M.; Neves, I.C. Study of silver species stabilized in different microporous zeolites. Microporous Mesoporous Mater. 2013, 181, 83–87. [Google Scholar] [CrossRef]
- Salavati-Niasari, M. Synthesis and characterization of 18-and 20-membered hexaazamacrocycles containing pyridine manganese (II) complex nanoparticles dispersed within nanoreactors of zeolite-Y. Polyhedron 2009, 28, 2321–2328. [Google Scholar] [CrossRef]
- Rivera-Garza, M.; Olguín, M.T.; García-Sosa, I.; Alcántara, D.; Rodríguez-Fuentes, G. Silver supported on natural Mexican zeolite as an antibacterial material. Microporous Mesoporous Mater. 2000, 39, 431–444. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, Y.; He, P.; Dong, F.; Xia, Y.; He, Y. Antibacterial zeolite with a high silver-loading content and excellent antibacterial performance. RSC Adv. 2014, 4, 5283–5288. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Zhang, Y.; Xiong, L.; Yan, Y.; Boopathy, R.; Mulchandani, A. Bactericidal and ammonia removal activity of silver ion-exchanged zeolite. Bioresour. Technol. 2012, 117, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kwakye-Awuah, B.; Williams, C.; Kenward, M.A.; Radecka, I. Antimicrobial action and efficiency of silver-loaded zeolite X. J. Appl. Microbiol. 2007, 104, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.J.; Mauricio, J.E.; Paredes, A.R.; Gamero, P.; Cortés, D. Antimicrobial properties of ZSM-5 type zeolite functionalized with silver. Mater. Lett. 2017, 191, 65–68. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F.; Catalano, M.; Taurino, A.; Licciulli, A.; Maffezolli, A.; Sannino, A. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. J. Mater. Sci. 2011, 22, 2005–2012. [Google Scholar] [CrossRef] [PubMed]




| Sample | Crystallinity (%) | Ag (XRF) (%) |
|---|---|---|
| Zeolite A | 100 | 0 |
| Zeolite A-Ag (1%) | 98 | 0.79 |
| Zeolite A-Ag (5%) | 86 | 1.57 |
| Zeolite A-Ag (10%) | 70 | 13.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, L.O.; Anaya, K.; Pergher, S.B.C. Synthesis of Antimicrobial Films Based on Low-Density Polyethylene (LDPE) and Zeolite A Containing Silver. Coatings 2019, 9, 786. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9120786
de Araújo LO, Anaya K, Pergher SBC. Synthesis of Antimicrobial Films Based on Low-Density Polyethylene (LDPE) and Zeolite A Containing Silver. Coatings. 2019; 9(12):786. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9120786
Chicago/Turabian Stylede Araújo, Luís Otávio, Katya Anaya, and Sibele Berenice Castellã Pergher. 2019. "Synthesis of Antimicrobial Films Based on Low-Density Polyethylene (LDPE) and Zeolite A Containing Silver" Coatings 9, no. 12: 786. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9120786