3.1. LiCoO2 (LCO)
Having a lamellar structure, LiCoO
2 (LCO) is the prototypal positive electrode material commonly used in Li-ion batteries that yields a practical specific capacity of 135 mAh·g
−1 in the voltage range from ~3.8 V (fully lithiated state) to ~4.2 V (charge state at Li
0.5CoO
2) [
46]. Since the early work in 1996 by Berkeley’s group [
47], numerous studies have been devoted to the growth of LiCoO
2 thin films prepared by the PLD technique due in large to their high electrochemical performances. Further investigations of dense and well-defined PLD films described the phase evolution during Li extraction and the kinetics of Li
+ ions in the host lattice, which eventually found applications in the fabrication of the cathode element in microbattery stacks [
48]. The two crystal forms, HT- and LT-LiCoO
2 phases, with the rock-salt (rhombohedral,
R-3
m space group) and spinel (cubic,
Fd3
m space group) structure, respectively, have been synthesized by pulsed-laser deposition. It was pointed out that the crystallographic texture for LCO films differs from one deposit technique to another, i.e., PLD versus sputtering, which influences the electrochemical properties due to the diffusion plane orientation [
49]. Julien et al. stated that well-crystallized PLD-grown LCO thin films with a single layered structure can be obtained at substrate temperatures (
Ts) as low as 300 °C [
3].
The first growth of single phase LCO films by the PLD method was realized by Antaya et al. [
4]. Films deposited on unheated stainless-steel substrates were amorphous but crystallized readily with heat treatment in air above 500 °C. Later, Striebel et al. [
47] demonstrated the promise of PLD-grown films as cathodes for rechargeable lithium cells. Crystalline (003)-textured LCO films with thicknesses ranging from 0.2 to 1.5 µm were prepared without postdeposition treatment, which displayed a specific capacity of films of 62 µAh·cm
2·µm
−1 and an Li diffusion coefficient of 1 × 10
−10 cm
2·s
−1. Highly dense LCO films were first elaborated by the PLD process using a KrF laser under oxygen flow rates of 30 sccm and the pressure was maintained at 260 Pa on (200)-textured F-doped SnO
2 on fa used silica substrate maintained at
Ts = 700 °C [
49]. As-prepared LCO thin films were (00l) textured and had a density of 85% of the single crystal. The charge–discharge profile of the films was typical of the LCO bulk and presented an ~18% capacity loss for a single cycle to 4.15 V. In the potential range of 4.14 to 4.19 V, the measured chemical diffusion coefficients ranged from 1.7 × 10
−12 to 2.6 × 10
−9 cm
2·s
−1 for as-deposited films and films annealed at 700 °C, respectively. Structural analysis of nanostructured LCO films prepared with PLD has been conducted by several research groups. Julien et al. [
3,
8,
50] analyzed changes of the stoichiometry (i.e., the absence of the Co
3O
4 amorphous phase) as a function of the growth conditions using Raman spectroscopy. The inclusion of Co
3O
4 impurity is detected by analysis of the Raman intensity of the
A1g modes. Impurity-free films exhibit a specific capacity as high as 195 mC·cm
−2·µm
−1 for polycrystalline films grown from an Li-rich target (i.e., excess of 15% Li
2O). The work by Okada et al. revealed that a decrease of the amount of inclusions can be obtained by a lower laser fluence and lower
Ts [
51].
Figure 2 presents the relationship between the impurity inclusions and growth conditions of PLD-grown LCO films established from spectroscopic Raman data. In this figure, the Co
3O
4 phase is grown under the conditions of high
PO₂, i.e., above the gray dashed line.
Ohnishi et al. [
52,
53] stated that suppression of the Co
3O
4 spinel phase can be ensured by the growth under a relatively low oxygen partial pressure. Zhang et al. [
54] discussed the effect of the deposition conditions on the structure and morphology. The advantages of the preferential orientation of LCO films prepared by PLD has been discussed by numerous groups with the conclusion that dense uniaxial textured (003)-oriented films are obtained by a well-chosen substrate [
6,
53,
55,
56,
57,
58,
59]. However, Xia et al. [
59] stated that the fast transport of Li
+ ions is obtained for LCO films with a random orientation, in contrast with the results obtained with films having (003)-preferred orientation. Contrastingly, Nishio et al. claimed an excellent electrochemical performance for epitaxially grown LCO (77-nm thick) with a (104)-orientation on a (100) Nb:SrTiO
3 substrate that exhibited a discharge capacity of 26 mAh·g
−1 even at high rates up to 100C [
60]. Huo et al. stated that the film orientation is strongly dependent on the thickness and size of grains and demonstrated that films structured with parallel (003) planes are grown for thicknesses up to 300 nm [
11]. Liu et al. showed that under certain PLD conditions, such as a high repetition rate of 35 Hz and low oxygen partial pressure of
PO₂ = 1 Pa, LCO films tend to grow LCO films with a random orientation [
61].
Epitaxial LCO thin films deposited on (001)-Al
2O
3 substrates remained in a single phase in a narrow range, 250 ≤
Ts ≤ 300 °C, whereas secondary phases appeared at
Ts > 300 °C, i.e., Co
2O
3, Co
3O
4, and LiCo
2O
4 [
5]. Xia et al. established that thin LCO films can be easily grown with a (003) orientation because of the lowest surface energy for the (003) plane, while the minimized strain energy in thick LCO films allows preferential (101) and (104) textures. It seems that this last type of orientation favors the electrochemical performance of the LCO cathode [
12]. The reduction of the laser fluence results in a decrease of the surface roughness of LCO films. With post annealing at 400 °C and optimized deposition conditions, LCO films exhibit an initial discharge capacity of 36 µAh·cm
−2·µm
−1 and a cycleability of 94% [
57]. Composition control was monitored to prepare stoichiometric LCO films using an Li-enriched target with a high-rate growth via an increase of the laser fluence to 0.29 J·cm
−2 and an adjustment of the
PO₂ to scatter the excess lithium. Ohnishi et al. showed that by using a Li
1.1CoO
2+δ target, the deposition of stoichiometric LCO with the highest crystallinity can be realized at the rate of 0.06 Å per pulse at the
PO₂ of 0.1 Pa and
Ts = 800 °C (
Figure 3) [
52,
62].
Recently, Nishio et al. claimed that a high deposition rate of 1.2 Å·s
−1 tends to form oxygen-deficient LCO films due to the destabilization of Co
3+ cations and showed that post-annealing in air cancels the impurity phase [
63]. Funayama et al. studied the effects of mechanical stress applied to LCO films (200 nm thick) deposited on Li-glass ceramic by the PLD method at 600 °C under a 20 Pa oxygen partial pressure for 1 h. Due to the lattice volume change, the generated electromotive force was 6.1 × 10
−12 V·Pa
−1 [
64]. The electrode behavior shows an increase of the discharge capacity from 10 to 40 mAh·g
−1 at a 2C rate with an increase of the
Ts from 600 to 750 °C, whereas a
Ts = 800 °C worsens the performance. Studies of the physico-chemistry of PLD-grown LCO thin films report the structural, surface morphology, optical, and electrical properties [
65,
66,
67].
The electrochemical properties, i.e., thermodynamics and kinetics, of lithium intercalation in PLD LCO thin films have been widely investigated by the structure–electrochemistry relationship [
59,
68,
69,
70]; structural evolution upon charge-discharge cycles [
71]; effect of doping by Ti, Al, and Mg [
49,
72,
73,
74]; characterization of the electrode/electrolyte interface [
75]; and lithium-ion kinetics vs. basal plane orientation [
76,
77,
78,
79,
80]. Highly (003)-oriented impurity-free LCO thin films grown by PLD on a stainless-steel substrate display an initial discharge capacity of 52.5 µAh·cm
−2·µm
−1 and a capacity loss of 0.18% per cycle at a moderate current density of 12.7 µA·cm
−2. These films show a very small lattice expansion upon charge, i.e., 0.09 Å for a charge of 4.2 V [
81].
Figure 4 shows the typical electrochemical features of PLD LCO thin films grown on an Si wafer maintained at different temperatures. Both the specific discharge capacity and the mid-voltage increased with increasing
Ts. For a film deposited at
Ts = 300 °C under
PO₂ = 15 Pa, the discharge capacity reached a value ~140 mC·cm
−2·µm
−1.
The electrochemical behavior of doped LCO thin films show that the voltage plateau at 3.65 V disappears in the charge curve of LiTi
0.05Co
0.95O
2 due to the doping effect, which cancels the semiconductor-metal-like transition of the LCO framework [
81]. The Al-doped LCO film (LiCo
0.5Al
0.5O
2) exhibits a steady increase in the voltage vs. Li extraction with the absence of a voltage plateau as observed in stoichiometric LCO films; however, such films suffered from a limited capability and an upper bound of the diffusion coefficient of Li (
D* = 9 × 10
−13 cm
2·s
−1) was observed [
49]. Recent studies report on the improved electrochemical behavior of surface-modified LCO films using lithium tantalate (LTaO) and lithium niobite (LNbO). Coating with LNbO preserves the LCO surface and decreases the interfacial resistance, which indicates fast lithium transport [
82]. LCO films modified by amorphous tungsten oxide (LWO) fabricated by PLD show a high capacity retention of
Qr = 80% at a high rate of 20C, against
Qr = 0% for bare LCO films cycled at the same C-rate. A slight increase of the superficial diffusion coefficient of Li
+ ions from 2.2 × 10
−13 and 3.0 × 10
−13 cm
2·s
−1 was also observed, owing to the surface modification [
83,
84,
85,
86]. Note that LWO as well as LNBO are lithium ion conductors, which act as an efficient buffer between the electrolyte and LCO cathode. The structural degradation of cycled LCO films was investigated by Raman spectroscopy over 400 cycles, showing microstructural modification due to nanocrystallization and phase separation [
87].
All-solid-state lithium microbatteries (SSLMB) using LiCoO
2 films have been developed using various inorganic solid electrolyte (SE) films, i.e., LiPON, Li
2S–P
2S
5, and amorphous Li
3PO
4. The thin-film battery with an electrochemical chain Li/amorphous Li
3PO
4/LCO/Pt shows a columnar-like LCO cathode (see the cross-sectional SEM image in
Figure 5a) [
88]. The excellent electrochemical performance is displayed in
Figure 5b. This microcell exhibited an increase in capacity of up to 240 µAh·cm
−2 when increasing the LCO thickness to 6.7 µm, which is 54% of the theoretical specific capacity of LCO (69 µAh·cm
−2·µm
−1). Shiraki et al. fabricated an SSLMB with an epitaxial LCO thin-film cathode (200 nm thick) by using PLD with a polycrystalline Li
1.1CoO
2 target ablated at a laser fluence of 1 J·cm
−2, LiPON solid electrolyte (2 µm thick), and Li film as the anode (0.5 µm thick) [
89]. The authors reported cyclic voltammograms with six redox peaks, which drastically changed upon cycling but did not display the galvanostatic charge–discharge profile of the SSLMB.
3.2. LiNiO2 (LNO)
PLD-grown thin films of lithium nickel oxide (LNO), i.e., Li
xNi
1−xO and stoichiometric LiNiO
2, are applied as electrochromic and/or battery electrodes. In an early work, Rubin et al. established the complex relationship of the surface morphology and chemical composition of Li
xNi
1−xO thin films vs. the deposition oxygen partial pressure, substrate temperature, and substrate–target distance as well [
90]. LNO films produced at
Ts < 600 °C immediately absorb CO
2 and H
2O when exposed to air, whereas they show long-term stability for
Ts = 600 °C. LNO film with a composition of Li
0.5Ni
0.5O (cubic rock-salt
Fd-3
m structure instead of the rhombohedral
R-3
m structure for LiNiO
2) was obtained under a deposition atmosphere of
PO₂ = 60 mTorr. This film (150-nm thick) showed excellent electrochemical reversibility as an electrochromic item in the range of 1.0 to 3.4 V vs. Li
+/Li. An electrochromic device using WO
3 as the opposite electrode and PEO/LiTFSI as the solid polymer electrolyte (250-µm thick) showed an optical transmission range of ≈70% at 550 nm. Bouessay et al. optimized the PLD conditions to prepare NiO films, i.e.,
PO₂ = 0.1 mbar and
Ts = 25 °C, and analyzed the electrochromic reversibility associated with the Ni
3+/Ni
2+ redox couple [
91]. Using a laser fluence of 1 to 2 J·cm
−2, which corresponded to an ablation rate of 0.9 Å·s
−1, NiO films with a cubic rock-salt structure (
Fm-3
m space group) were formed. Porous PLD NiO films were prepared using nickel foil as the target in a low oxygen atmosphere (
PO₂ = 50 Pa) [
92,
93] and were applied as the electrode for supercapacitors, showing a high specific capacitance of 835 F·g
−1 at a 1 A·g
−1 current density.
Similarly to LCO, the PLD growth of stoichiometric LiNiO
2 with an α-NaFeO
2 layered structure requests an Li-enriched target, i.e., LiNiO
2 + 15%Li
2O. López-Iturbe and coworkers attempted to avoid Li loss by using an Ar atmosphere of
PAr = 10 mTorr and laser fluence of 15 J·cm
−2 [
94], while Rao et al. introduced pure oxygen (
PO₂ = 0.1 Torr) in the PLD chamber and ablated the target at a laser fluence of 10 J·cm
−2 [
95]. The LNO films prepared at
Ts = 700 °C exhibited an initial discharge capacity of 175 mC·cm
−2·µm
−1. Yuki et al. used, as the oxygen evolution reaction (OER), electrocatalysts, which were prepared LNO films from a target composed of Li
2O and NiO
2 sintered at 1000 °C for 8.5 h in air ablated by a Nd:YAG laser operating at 532 nm [
96]. Recently, PLD Li
xNi
2−xO
2 thin films with 0.15 ≤
x ≤ 0.45 deposited on a glass substrate under a pressure of 0.1 Pa and annealed at 350 °C were grown by PLD with an LiNiO
2 structure. The films appeared to be entirely made of particles even in the cross-section (grain size of 95 nm for
x = 0.45). The average surface roughness estimated from the AFM measurements decreased with an increasing
x, reaching a value of 0.615 nm for
x = 0.45 [
97].
3.3. LiNi1−yCoyO2 (NCO)
The LiNi
1−yCo
yO
2 (0 <
y <1) system with a layered α-NaFeO
2 structure belongs to a LiCoO
2-LiNiO
2 solid solution with a higher reversible capacity than LCO and better cycleability than LNO. Among these substituted oxides, Ni-rich LiNi
0.8Co
0.2O
2 (NCO) has been identified as one the most attractive cathodes [
98]. In this context, several works investigated the growth of NCO thin films using pulsed-laser deposition. Dense PLD NCO films grown at
Ts > 400 °C exhibited a gravimetric density of 4.8 g·cm
−3 [
99]. Ramana et al. grew NCO films deposited on an Ni foil substrate at temperatures of 25 ≤
Ts ≤ 500 °C under
PO₂ = 6 to 18 Pa from Li-rich ceramic (15 mol% Li
2O excess to avoid NiO or Co
3O
4 impurity phases) [
100]. At
Ts ≤ 300 °C, the PLD film showed the highest intensity of the (00
l) reflection, which indicates that the
c-axis was normal to the film surface. The XRD (003) diffraction peak at 2θ = 18.5° corresponds to an interplanar spacing of 0.145 nm. Phase diagram mapping (
Figure 6) was proposed to highlight the effect of the growth temperature on the microstructure of PLD LiNi
0.8Co
0.2O
2 films. Galvanostatic titration carried out at a rate of
C/30 in the potential range of 2.5 to 4.2 V showed a discharge capacity of 82 µAh·cm
−2 µm
−1, which compares with the theoretical value of 136 µAh·cm
−2·µm
−1 (490 mC·cm
−2·µm
−1) [
101].
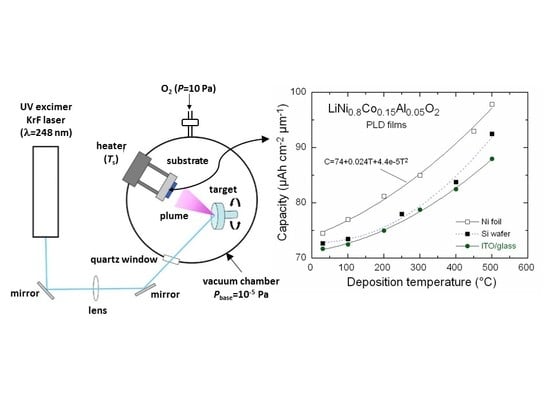
Hirayama et al. fabricated NCO films using the standard PLD conditions (Φ = 100–220 mJ,
PO₂ = 3.3 Pa, target composition Li/Ni(Co) = 1.3, and
Ts = 600–650 °C) at a deposition rate of 0.3 nm·min
−1 on an oriented SrTiO
3 (STO) substrate. Microstructural analysis shows a misfit of ca. 5% and roughness of 1 to 3 nm for the film grown with an in-plane orientation at
Ts = 600 °C. AFM imaging revealed the surface modification for films cycled in the voltage range of 2 to 5 V [
102]. PLD NCO films (0.62 µm thick) were electrochemically characterized by galvanostatic titration (GITT), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS). Films grown at
Ts = 600 °C under
PO₂ = 13 Pa with a laser fluence of Φ = 450 mJ per pulse exhibited an average specific capacity of ~60 µAh·cm
−2·µm
−1 and the Li
+ diffusion coefficient varied from 3 × 10
−13 to 2 × 10
−10 cm
2·s
−1. After 100 cycles, the electrode showed a capacity retention of 85% [
103]. The kinetics of the Li-ion intercalation in PLD NCO films grown on an Nb-doped STO substrate at
Ts = 600 °C under
PO₂ = 3.3 Pa were investigated by EIS [
104]. Nyquist plots showed changes of the electrode impedance as a function of the Li extraction/insertion with a larger value at a potential of 4.2 V. Baskaran et al. prepared NCO films on Pt and Si substrates heated at
Ts = 500 °C under a low oxygen partial pressure of 0.21 Pa [
105]. The 40-min deposited films (120-nm thick) displayed a specific discharge capacity of 69.6 µAh cm
−2·µm
−1 (145 mAh·g
−1) after 10 cycles. Based on these results, a Li-ion microbattery was fabricated with a LNCO/Li
3.4V
0.6Si
0.4O
4(LVSO)/SnO configuration with a thickness of ~1.5 μm. Such a microcell delivered a capacity of 16.1 µAh·cm
−2·µm
−1 after 20 cycles.
3.9. LiMn2O4 (LMO)
Since the early report in 1996 [
47], numerous studies have been devoted to LiMn
2O
4 (LMO) thin films grown by laser ablation. A spinel structure (
Fd3
m space group) was successfully prepared using different PLD conditions and applied as a positive electrode in thin-film lithium microbatteries (see, for example, [
121]). Most prior works focused on the fundamental properties of PLD-grown LMO cathode films, aiming to deposit the highly structured and porous morphology required for a good operating electrode [
7,
122,
123,
124,
125,
126,
127,
128,
129,
130,
131,
132,
133,
134,
135,
136]. Morcrette et al. analyzed PLD film composition as a function of
Ts and
PO₂ using the Rutherford backscattering method and nuclear reaction analysis [
122]. A stoichiometric LMO film was obtained at
Ts = 500 °C and
PO₂ = 20 Pa, while the Li/Mn ratio decreased with
Ts and increased with
PO₂. The film of Li
0.6Mn
2O
3 (20% oxygen loss) was obtained under vacuum. The Mn
3O
4 + MnO and Mn
3O
4 + LiMn
2O
4 mixed phases were successively grown in the range of 0.01 ≤
PO₂ ≤ 1 Pa. Julien et al. defined the conditions of the disposition of LMO films grown on Si substrates. Impurity-free well-crystallized samples with a crystallite size of 300 nm were obtained at
Ts as low as 300 °C and
PO₂ = 10 Pa using an Li-enriched target (15% Li
2O excess) to avoid Li deficiency in the film [
123]. The electrochemical features of the Li cell showed a specific capacity as high as 120 mC·cm
−2·µm
−1 in the voltage range of 3.0 to 4.2 V vs. Li
+/Li, which was attributed to the high degree of film crystallinity [
7,
123]. Simmen et al. studied the relationship between Li
1+xMn
2O
4−δ thin films and Li excess in the target and concluded that a film deposited from a composite target of Li
1.03Mn
2O
4-δ + 7.5 mol% Li
2O was the best, exhibiting a discharge capacity of 42 µAh·cm
−2·µm
−1 [
127]. Various substrates were successfully used for the epitaxial growth of LiMn
2O
4 (LMO) spinel thin films, such as Pt, Si, Au, MgO, Al
2O
3, and SrTiO
3. Gao et al. [
128] reported a detailed mechanism of the epitaxial LMO film/substrate (current collector) interface formation. A coherent hetero-interface was formed with the substrate but a tetragonal Jahn–Teller distortion was observed, induced by oxygens’ non-stoichiometry and the lattice misfit strain. PLD epitaxial LMO thin films were deposited on oriented Nb:SrTIO
3 substrates maintained at 950 °C from a sintered target with 100 wt.% excess Li
2O with different surface morphologies and orientations, such as (100)-oriented pyramidal, (110)-oriented rooftop, or (111)-oriented flat structure. The pyramidal-type LMO was cycled at a 3.3C rate, demonstrating a specific capacity of 90 mAh·g
−1 after 1000 cycles [
129]. Using oriented substrates, i.e., (111)Nb:SrTiO
3 (STO) and (001)Al
2O
3 single crystal, LMO films were grown with the (111) orientation under the following synthesis conditions: Li-enriched target (Li/Mn = 0.6),
Ts = 650 °C, and
PO₂ = 30 Pa [
130]. Electrochemical tests emphasized the interactions between the films and substrate, and showed a plateau voltage at 3.6 to 3.8 V for LMO/STO and 3.8 to 4.1 V for LMO/alumina. Canulescu et al. [
131] investigated the mechanisms of laser-plume expansion during the PLD of LMO films under
PO₂ ranging between 10
−4 and 20 Pa. The Li deficiency occurs as a result of the different behavior of the species at elevated
Ts. Hussain et al. [
132,
133] obtained highly oriented LiMn
2O
4 thin films on oriented Si substrates heated in the range of 100 ≤
Ts ≤ 600 °C under
PO₂ = 10 Pa and with a laser fluence of Φ =10 J·cm
−2. Grains with a spherical shape (around 230 nm in diameter) changed to a flake-like structure at
Ts = 600 °C (
Figure 8a). The grain size varied almost linearly with the substrate temperature (
Figure 8b).
By applying an elevated-temperature PLD technique, Tang et al. [
134] studied the influence of the substrate temperature (
Ts) and the oxygen partial pressure (
PO₂) on LMO film crystallinity. LMO thin films deposited on Si (001)/0.2 µm-SiO
2 substrates at 575 °C under 13 Pa oxygen had a flat and smooth surface and exhibited mainly a (111) out-of-plane preferred texture (
Figure 9a). Such films of the 300 nm thickness showed a very dense cross-section (density ~4.3 g·cm
−3) (see
Figure 9b) [
135]. The effect of stoichiometric deviations on the electrochemical performance of an LMO thin-film cathode was investigated by Morcrette et al. [
136], while the kinetics of Li
+ ions in the LMO thin-film framework were documented by Yamada and coworkers [
137]. A high-activation barrier of 50 kJ·mol
−1 for Li-ion transfer was identified at the electrode/electrolyte interface for films deposited at
Ts = 700 °C and
PO₂ = 27 Pa. Albrecht et al. [
138] reported the minimum crystallization temperature of spinel LMO thin films in a narrow annealing temperature range of around 700 °C. Electrochemical tests carried out with the galvanostatic cycling with the potential limits (GCPL) method proved that Li-ions are (de-)intercalated in different tetrahedral sites for which the processes occur at potentials that are slightly shifted by
U ≈ 100 mV, which is similar to the previous results by Julien et al. [
7].
Studies of the structure and electrochemical reactivity of heteroepitaxial LiMn
2O
4/La
0.5Sr
0.5CoO
3 (LMO/LSCO) bilayer thin films deposited on crystalline SrTiO
3 substrates show that LSCO reduced the lattice misfit strain with the substrate and favored a lower LMO surface roughness. However, a decrease of the electrical conductivity occurred during the electrochemical test (after first cycle) due to the lattice oxygen loss at the outermost layer (40 nm) [
139]. Tang et al. reported a comparative investigation of the structures, morphologies, and properties of Li insertion for LMO films with different crystallizations. At
Ts = 400 °C, LMO films consisted of nanocrystallites < 100 nm in size with rough surfaces that exhibited a discharge capacity of 61 µAh·cm
2·µm
−1 with a capacity loss of 0.032% per cycle up to 500 cycles, while for
Ts = 600 °C and
PO₂ = 10 Pa, highly crystallized films showed an initial discharge capacity of 54.3 µAh·cm
2·µm
−1 [
134]. The intrinsic properties of PLD-grown LMO have been investigated by several techniques. Electrical measurements of LMO films showed that the conductivity is sensitive to
Ts, as the activation energy that followed the Mott’s rule increased with
Ts up to
Ea = 0.64 eV at
Ts = 600 °C [
133]. Singh et al. characterized the crystallinity and texture of LMO films deposited at
Ts = 650 °C. Here, (111)-oriented films were grown on a doped Si substrate, while films deposited on a stainless-steel substrate exhibited a (001) orientation [
140]. The thermo-power (or Seebeck coefficient) of PLD LMO films was reported to be 70 µV·K
−1 [
141].
PLD LMO films were subjected to an overcharge (5 V vs. Li
+/Li), which did not modify the structure and preserved the well-resolved voltage peaks at 4.1 and 4.2 V, while an overdischarge (2 V vs. Li
+/Li) led to a loss of capacity due to the structural disorder associated with the tetragonal transition, i.e., Jahn–Teller distortion [
142]. Singh patented the fabrication of PLD Li
1−xMyMn
2−2zO
4 films, where
M is a doping element (
M = Al, Ni, Co, Cr, Mg, etc.) and
x,
y, and
z vary from 0.0 to 0.5 [
143]. These defective spinel structures enhanced the oxygen content as compared to LiMn
2O
4 crystal. In particular, the oxygen-rich Li
1−δMn
2−δO
4 films were superior cathode films, leading to excellent rechargeable battery performances. It is claimed that a high discharge rate of 25C produces only a 25% capacity loss and a specific capacity >150 mAh·g
−1 remains after 300 cycles. Rao et al. reported the preparation of well-crystallized LMO films at a high substrate temperature of
Ts = 700 °C and
PO₂ = 13 Pa that delivered a capacity of 133 mC·cm
−2·µm
−1 at a very slow C/100 rate [
144]. Several workers reported the evolution of the thin-film electrode/electrolyte interface, as the planar form of the film is the ideal geometry for such investigations [
145,
146,
147,
148]. Room temperature impedance measurements were carried out to identify the formation of the solid electrolyte interface (SEI) layer on a PLD LMO film cathode and the degradation mechanism during cycling in an aprotic electrolyte containing LiPF
6 salt. A reversible disproportionation reaction was suggested with the formation of the Li
2Mn
2O
4 and λ-MnO
2 phases at the surface [
146]. Using epitaxial-film model electrodes, Hirayama studied the surface reaction and the formation of the SEI layer and the interfacial structural reconstruction during an initial battery process using in situ surface X-ray diffraction and reflectometry [
147]. TEM images confirmed the surface reconstruction that occurred during the first charge, i.e., when a potential was applied. After 10 cycles, the SEI layer was observed on both the (111) and (110) surfaces and Mn dissolution appeared at the (110) surface [
148]. Inaba et al. [
149] investigated the surface morphology evolution of PLD LMO thin films grown on a PT substrate at
Ts = 600 °C by electrochemical scanning tunneling microscopy (STM) with voltage cycling in the range of 3.5 to 4.25 V. The original LMO grains of 400 nm in size coexisted with small particles 120 to 250 nm in size, which appeared after 20 cycles and decreased to ~70 nm after 75 cycles through a kind of dissolution/precipitation process. LMO thin-film electrodes with a grain size of <100 nm deposited on a stainless steel substrate at
Ts = 400 °C under a 26 Pa oxygen partial pressure displayed an excellent capacity of 62.4 µAh·cm
−2·µm
−1 when cycled at a 20 µA·cm
−2 current density in the voltage range of 3.0 to 4.5 V. A very low capacity fading was recorded for up to 500 cycles at 55 °C. Li
+-ion diffusion coefficients evaluated from EIS measurements were around 2.7 × 10
−12 cm
2·s
−1 for an electrode charged at 4.0 V and 2.4 × 10
−11 cm
2·s
−1 for 4.2 V [
150]. Xie et al. [
151] investigated the Li
+-ion transport in LMO thin films (~100 nm thick) grown on Au substrates at 600 °C at a deposition rate of 0.14 nm·min
−1. The chemical diffusion coefficients determined by the EIS, GITTm, and PITT methods were in the range of 10
−14 to 10
−11 cm
2·s
−1 in the voltage range of 3.9 to 4.2 V.
Table 5 lists some typical results on the kinetics of Li
+ ions in pulsed-laser deposited LMO thin films.
The electrochemical behavior of Li-rich spinel Li
1.1Mn
1.9O
4 thin films grown by PLD on an Au substrate was reported by several workers [
156,
157]. The best performance was reported at a discharge current density of the 36C-rate for LMO films deposited for 30 min in
PO₂ = 30 Pa and
Ts = 600 °C with an Nd:YAG laser (266 nm) adjusted to an energy fluence of 1 J·cm
−2 [
156]. Nanocrystalline LMO films with grains less than 100 nm were deposited on a stainless-steel substrate at
Ts = 400 °C and
PO₂ = 26 Pa using a PLD pulse power of 100 mJ at the frequency of 10 Hz. The film cycled over 100 cycles delivered a specific capacity of 118 mAh·g
−1 at a current density of 100 A·cm
−2 [
157]. Using reflectometry measurements, Hirayama et al. [
158] studied the structural modifications at the electrode/electrolyte interface of a lithium cell, in which the LMO electrodes were prepared as epitaxial films by the PLD method with different orientations. The respective orientation of the LMO film corresponded to that of the substrate plane, i.e., the (111), (110), and (100) planes of the SrTiO
3 substrate. No density change was observed for the (110) and (100) planes, whereas a defect layer was detected in the (111) plane. ZrO
2-modified LiMn
2O
4 thin films prepared via PLD consisting of amorphous ZrO
2 formed on the grain boundary and the outer layer of the LMO matrix [
159]. The high capacity retention of 82% after 130 cycles of films of
xZrO
2-(1−
x)LiMn
2O
4 (
x = 0.025) monitored at the 4C rate was attributed to the decrease of the charge transfer resistance (
Rct).
Epitaxial LiMn
2O
4/La
0.5Sr
0.5CoO
3 (LMO/LSCO) bilayer thin films with sub-nano flat interfaces were deposited on (111)-oriented STO substrates at
Ts = 650 °C in
PO₂ = 4 Pa. After the first charge–discharge cycle, the decrease of the electrical conductivity of the LSCO buffer layer due to lattice oxygen loss induced capacity fading [
139]. The PLD growth of a multilayer LMO thin film electrode demonstrated the compensation of lithium loss during deposition [
160]. Such a sample prepared in the PLD conditions (
Ts = 650 °C, Φ = 530 mJ·cm
−2, and
PO₂ = 1.3 Pa) showed the typical two pairs of voltammetry peaks at 0.82 and 1.02 V vs. Ag/AgCl in an aqueous cell. Kim et al. [
161] prepared a Li
0.17La
0.61TiO
3/LiMn
2O
4 (LLTO/LMO) hetero-epitaxial electrolyte/electrode by PLD with an energy fluence of Φ = 1.7 J·cm
−2 in a
PO₂ = 6.6 Pa atmosphere. The typical herostructure is composed of a 17.5-nm thick LMO, 7-nm thick interfacial layer, and 26.5-nm thick LLTO deposited on a (111)-oriented STO substrate. Voltammograms of the first and second cycles displayed redox peaks around 3.8 V attributed to an oxygen-deficient LMO and around 4.0 and 4.2 V, which are the typical redox voltages of the LMO spinel. Suzuli et al. [
162] prepared multi-layer epitaxial LiMn
2O
4/SrRuO
3 (LMO/SRO) thin film electrodes deposited for 30 min via PLD on (111)STO substrates heated at 650 °C using an Li
1.2Mn
2O
4 target in
PO₂ = 6.6 Pa. The LMO(33 nm)/SRO(38 nm) film exhibited a discharge capacity of 125 mAh·g
−1 with the typical plateau regions of LMO in the charge–discharge reaction. Yim et al. substituted Sn for Mn in PLD LMO thin films [
163]. The LiSn
x/2Mn
2−xO
4 films were prepared on a Pt/Ti/SiO
2/Si(100) substrate in the conditions of
Ts = 450 °C,
PO₂ = 26.7 Pa, Φ = 4.6 J·cm
−2, 10 Hz pulse frequency, and 4 cm target-substrate distance. XPS and EXAFS measurements showed that Sn
2+ cations replace Mn
3+ ions, which resulted in an increase of the valence of Mn in the spinel lattice. A high specific capacity of ∼120 mAh·g
−1 and cycleability with a capacity retention >81% at the 4C rate after 90 cycles was attributed to the Mn-deficient structure. A multi-layer PLD process was utilized to deposit LMO films (90 nm thick) on Si-based substrates coated with Pt as the current collector [
164]. A reversible capacity of 2.6 µAh·cm
−2 (corresponding to a specific capacity of ≈28 µAh·cm
−2·µm
−1 or 66 mAh·g
−1 assuming a dense film with 4.3 g·cm
−3) was reached at an extremely high current density of 1889 µA·cm
−2 (equivalent to the 348C rate) with a capacity retention of 86% over 3500 cycles. A significant non-diffusion-controlled contribution (pseudocapacitive-like) was evidenced by cyclic voltammetry; however, the two typical voltage plateaus in the GCD of LMO (around 4 V) indicates that the faradaic redox reaction is the main process. For an easy comparison,
Table 6 lists the electrochemical properties of PLD-prepared LMO thin film electrodes.
3.10. LiNi0.5Mn1.5O4 (LNM)
LiNi
xMn
2−xO
4 is a substituted oxide spinel that operates at high voltages >4.5 V upon Li extraction. Substituted spinel films of Li
xMn
2−yMyO
4 where
M = Ni, Co and 0 ≤
y ≤ 0.25 as-prepared with a crystalline morphology (0.3 µm thick) showed the typical features of high-voltage electrodes without carbon additive or binder materials in the range of 2.0 to 5.8 V vs. Li
+/Li [
165]. Cyclic voltammetry showed that: (i) PLD LiMn
1.9Ni
0.1O
4 films charged at 5.7 V do not show capacity fading; (ii) LiMn
2O
4 and LiMn
1.75Co
0.25O
4 films present a good stability to 5.6 and 5.4 V vs. Li
+/Li, respectively; and (iii) below 3 V the films exhibit the typical Jahn–Teller distortion. The compound, LiNi
0.5Mn
1.5O
4 (LNM), is more interesting because of the oxidation state of cations: Ni
2+ can be oxidized twice (i.e., 2e
− transfer) during charge while Mn
4+ is electrochemically inactive. Xia et al. showed that laser-ablated LNM films 0.3 to 0.5 µm thick deposited on a stainless steel substrate heated at 600 °C under an oxygen partial pressure of 26 Pa exhibit excellent capacity retention (i.e., ~120 mAh·g
−1 after 50 cycles) in the voltage range of 3 to 5 V vs. Li
+/Li [
166]. Well-crystallized oxygen deficient LiMn
1.5Ni
0.5O
4−δ films deposited by PLD at a controlled fluence of Φ = 2 J·cm
−2,
Ts = 600 °C, and
PO₂ = 26 Pa for 40 min exhibited a stepwise voltage profile near 4.7 V and a small plateau in the 4 V region. These disordered spinel structures had a stable specific capacity of 55 µA h cm
−2·µm
−1 in the voltage range of 3 to 5 V vs. Li
+/Li. The good rate capability was due to the high kinetics for Li diffusion in the range of 10
−12 to 10
−10 cm
2 s
−1 measured by the potentiostatic intermittent titration technique (PITT). These values are comparable to that of layered LCO [
167,
168]. Epitaxial LNM films were grown on single-crystal oriented SrTiO
3 (STO) substrates from an Li-enriched target with Li/(Ni + Mn) = 0.6. The film orientation, i.e., (100)-, (110)-, and (111)-oriented, were the replica of those of the STO substrates [
169]. Depending on the film orientation and thickness, the discharge profiles exhibited two to three plateaus around 3.9, 4.5, and 4.7 V vs. Li
+/Li, which were attributed to the Mn
3+/Mn
4+, Ni
2+/Ni
3+, and Ni
3+/Ni
4+ redox couples, respectively. Note that the emergence of the Mn
3+/Mn
4+ redox couple was due to the introduced oxygen vacancies [
170].
Other 5-V class cathode thin films include LiCoMnO
4. PLD LiCoMnO
4 films prepared under standard conditions (i.e.,
Ts = 500 °C,
PO₂ = 20–100 Pa, and Φ = 2 J·cm
−2) had a composition of Li:Co:Mn = 0.99:0.98:1. These films were tested in the voltage range of 3.0 to 5.5 V vs. Li
+/Li in SSMB consisting of Li/Li
3PO
4/LiCoMnO
4 fabricated on Pt/Cr/SiO
2 substrates [
171]. Cyclic voltammetry showed that the higher capacity in the 5-V region was obtained for the film grown under
PO₂ = 100 Pa (
Figure 10). A specific discharge capacity of 90 mAh·g
−1 remained after 20 cycles. Epitaxial Li
0.92Co
0.65Mn
1.35O
4 film with a cubic spinel structure was grown on a SrTiO
3(111) single-crystal substrate using a layer-by-layer technique, which consisted of repeating a Li
1.2Mn
2O
4/Li
1.4CoO
2 deposition process at
Ts = 650 °C and
PO₂ = 6.6 Pa using a KrF excimer laser (λ = 248 nm) [
172]. For a film area of 0.7 mm
2, thickness of 33.4 nm, and density of 4.38 g cm
−3, the specific discharge capacity was 340 mA·g
−1 at the second cycle. A capacity retention of 80% was observed after 20 cycles.
3.12. LiMPO4 (M = Fe, Mn) Olivines
LiFePO
4 (LFP) thin-film electrodes have been successfully fabricated by pulsed-laser deposition [
156,
177,
178,
179]. It was shown that, due to the film thickness and carbon content, the electrochemical performances are very sensitive, i.e., electronic conductivity and Li-ion diffusion. Iriyama et al. reported the PLD growth of olivine structured LFP thin films and their electrochemical properties characterized by cyclic voltammetry and charge–discharge tests [
180,
181]. The typical olivine features were evidenced by CV measurements in the range of 2.0 and 5.0 V vs. Li
+/Li, i.e., a single couple of anodic and cathodic peaks at ~3.4 V. Song et al. synthesized PLD LFP films with a low carbon content (<1 wt. %) on stainless steel substrates utilizing an Ar atmosphere [
182]. The 75-nm thick films showed reversible cycling of more than 80 mAh·g
−1 after 60 cycles. Furthermore, 156-nm thick films grown using a target–substrate distance reduced to 5 cm had a layered surface texture and delivered more than 120 mAh·g
−1 with a good capacity retention. LFP thin films with a needle-like morphology were prepared by an off-axis PLD technique [
183]. The effect of the substrate on the structure and morphology was examined by Palomares et al. for PLD film deposited under argon gas kept at a pressure of 8 Pa [
184]. Stainless steel was demonstrated to be the best substrate for the single-phase olivine (
Pnma space group) with a temperature set at 500 °C.
LiFePO
4 deposited by pulsed-laser deposition proved to be effective as a thin film electrode. Tang et al. stated that a well-crystallized pure olivine phase was grown using optimized deposition parameters (
Ts = 500 °C,
PAr = 20–30 Pa, pulse power of 120 mJ, pulse frequency of 10 Hz, λ = 248 nm) [
185]. An electrochemical capacity of 38 µAh cm
−2·µm
−1 at the C/20 rate (36 µAh·cm
−2·µm
−1 at a rate of C/4) was measured at 25 °C. High substrate temperatures (500 ≤
Ts ≤ 700 °C) favored the presence of Fe
3+ impurities, i.e., Li
3Fe
2(PO
4)
3 and Fe
4(P
2O
7)
3. In a second article, the same group analyzed the kinetics of Li
+ ions in PLD LFP films using CV, GITT, and EIS measurements [
179]. CV data provided average
D* values of 10
−14 cm
2·s
−1, while
D* deduced from both GITT and EIS techniques was in the range of 10
−14 to 10
−18 cm
2·s
−1. A maximum
D* value was observed at
x = 0.5 for Li
xFePO
4. Lu et al. prepared different composite thin films, i.e., LiFePO
4–Ag and LiFePO
4–C, with the aim of enhancing the electronic conductivity [
177,
178]. It was found that films grown with 2 mol% carbon and annealed at 600 °C for 6 h had an improved coulombic efficiency. Well-crystallized olivine-type structure LFP films were obtained by PLD coupled with high temperature annealing of 650 °C. The first discharge capacity was 27 mAh·g
−1 with a retention of only 49% after 100 cycles. The low reversible capacity and poor cycling performance was attributed to the existence of an Fe
2O
3 impurity produced by the high temperature treatment and poor intrinsic conductivity [
186]. Sauvage et al. published several reports on the electrochemical properties of PLD LFP thin films grown in different configurations [
187,
188,
189,
190]. First, it was shown that well-crystallized and homogeneous 300-nm thick LFP films deposited on Pt-capped Si substrates have intrinsic Li insertion properties evaluated both in aqueous and non-aqueous electrolytes, i.e., voltage plateau at 3.42 V vs. Li
+/Li [
187]. Second, the influence of the film thickness was studied in the range of 12 to 600 nm [
188]. Third, the effect of the texture on the electrochemical performance was analyzed for PLD films deposited on a polycrystalline α-Al
2O
3 substrate coated with a 20-nm thick Pt layer from an LiFePO
4 pellet as the target. The standard PLD conditions were used (i.e., (Φ = 2 J·cm
−2,
PAr = 8 Pa,
Ts = 600 °C) [
189]. Finally, the electrochemical stability of LFP films was analyzed as a function of the exposition to the most common lithium salt and for different current collectors (i.e., Si, Pt, Ti, Al, and (304)-stainless steel) [
190]. A 270-nm thick film tested by CV at a 2 mV·s
−1 scanning rate in 1 mol·L
−1 LiClO
4 in EC/DMC solution delivered a specific capacity of 1.52 µAh cm
−2 after 150 cycles. Recently, Raveendran et al. reported the properties of FeSe and LiFeO
2/FeSe bi-layers prepared by PLD as cathode materials [
191]. Mangano-olivine LiMnPO
4 (LMP) thin films were fabricated on Pt-coated SiO
2 glass substrates using PLD parameters, e.g., Φ = 1.58 J·cm
−2,
Ts = 400–700 °C, and
PAr = 2–100 Pa [
45]. LMP films (50-nm thick, 0.09 cm
2 area) were applied in Li/Li
3PO
4/LiMnPO
4 microbatteries for 500 cycles. From the CV measurement, a capacity of 28 mAh·g
−1 at 20 mV min
−1 was reported.
3.13. V2O5
Another candidate material for the cathodes of microbatteries is V
2O
5, in which about 1 mol of Li
+ ions can be inserted and extracted without the phase transformation of V
2O
5, leading to a theoretical specific capacity of 147 mAh·g
−1. Due to its stable layered structure and its ability to accommodate large amounts of Li ions, V
2O
5 has been widely studied for the development of electrochromic displays, color memory devices, and lithium-battery cathodes [
192]. Extensive works have evidenced the advantages of PLD for the preparation of V
2O
5 films with a good reproducible stoichiometry similar to the target material [
193,
194,
195,
196,
197,
198,
199,
200,
201,
202,
203,
204,
205,
206,
207]. The first work related to the growth of V
2O
5 thin film by PLD as an electrode for a thin-film lithium battery was reported by the National Renewable Energy Labs (USA) [
193] followed by Julien’s group [
194,
195]. A major advantage of laser ablation deposits is that it is possible to prepare thin layers of crystallized V
2O
5 under oxygen at a relatively low temperature of 200 °C [
193]. The growth mechanism of PLD V
2O
5 thin films has been proposed by Ramana and coworkers [
196]. It was reported that the grain size, surface roughness, and global morphology are highly sensitive to the nature and temperature of the substrate for films deposited in an oxygen partial pressure of
PO₂ = 13 Pa. The functional influence of the growth temperature on the grain size for films deposited onto various substrates was also evidenced. Two main features should be pointed out: (i) The exponential variation of the grain size over the substrate temperature range of 25 to 500 °C; (ii) the variation is dependent on the substrate material, which is larger for the Si(00) wafer. McGraw et al. reported that pulsed-laser deposited V
2O
5 films can be grown on a number of low-cost substrates, including SnO
2-coated glass, on which highly textured (001) films are obtained at
Ts = 500 °C under
PO2 in the range of 0.2 to 0.5 Pa [
52,
197,
198]. PLD thin films of V
2O
5 were prepared for applications in lithium batteries using a ceramic V
2O
5 target and a KrF laser of a wavelength of 248 nm. Depending on the temperature of the substrates and the oxygen pressure during deposition, amorphous or crystallized layers are obtained. PLD-grown amorphous films exhibited a low capacity loss of ~2% over 100 discharge–charge cycles in the voltage range 4.1 to 1.8 V compared to 20% for crystalline film [
45,
199]. Thin layers of V
2O
5 were also prepared using a V
2O
3 target [
200]. By making deposits at 200 °C with the same V
2O
3 target, amorphous layers were obtained in the absence of oxygen and layers crystallized in the presence of oxygen. Madhuri et al. [
201] reported the successful crystallization of laser-ablated V
2O
5 thin films at
Ts = 200 °C. These films were grown in the orthorhombic structure and exhibited a predominant (001) orientation. The growth of crystalline thin dense films without post-deposition annealing was claimed and the good electrochemical performance of PLD films was demonstrated. Iida et al. [
202] addressed the electrochromic properties of V
2O
5 films deposited onto ITO glass as a function of the PLD parameters. The film recrystallization occurred in the range of 400 ≤
Ts ≤ 500 °C and the best morphology was obtained for
PO₂ = 13.3 Pa. McGraw et al. deposited thin films of V
2O
5 for applications in lithium batteries using a ceramic V
2O
5 target and a KrF laser, with a wavelength of 248 nm. Depending on the temperature of the substrates and the oxygen pressure during deposition, amorphous or crystallized layers were obtained [
193,
199]. Thin layers of V
2O
5 were also prepared using a V
2O
3 target. By making deposits at 200 °C with the same V
2O
3 target, amorphous layers were obtained in the absence of oxygen and layers crystallized in the presence of oxygen. Stoichiometric amorphous V
2O
5 films can be grown onto substrates maintained at low temperatures (
Ts < 100 °C) using a sintered V
2O
5 target. Ramana et al. revealed that stoichiometric V
2O
5 films can be grown with a layered structure onto amorphous glass substrates at temperatures as low as 200 °C and an oxygen partial pressure of 100 mTorr [
203]. The onset of crystallization occurred at 200 °C with an activation energy of 0.43 to
n0.55 eV [
204]. Correlations between the growth conditions, microstructure, and optical properties were investigated for V
2O
5 thin films deposited over a wide substrate temperature range of 30 to 500 °C by Rutherford backscattering spectrometry (RBS), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and UV−vis−NIR spectral measurements. As shown in
Figure 11, the film grain size follows a power law of the substrate temperature and the optical energy bandgap decreases from 2.47 to 2.12 eV with the increase of
Ts from 30 to 500 °C [
205]. Bowman and Gregg investigated the effect of the applied strain on the resistance of V
2O
5 thin films grown from both metallic vanadium and a ceramic V
2O
5 target using a laser fluence of ~3.0 and ~1.5 J cm
−2, respectively [
208]. Deng et al. compared the growth of V
2O
5 films using femtosecond (f-PDL) and nanosecond (n-PDL) pulsed laser deposition using SEM, XRD, and Raman spectroscopy. Prior to annealing, f-PLD films showed a rougher texture and nano-crystalline character, while n-PLD films were much smoother and predominantly amorphous [
209].
The PLD growth conditions were refined by an analysis of the surface properties for the production of high-quality V
2O
5 films. The investigations were carried out by AFM, SEM, FTIR, and XRD. AFM measurements showed a surface roughness of ~12 nm with a Gaussian-like height distribution of surface grains for films deposited at
Ts = 200 °C under
PO₂ = 10 Pa [
210]. The local structure of 0.3-µm thick films grown on Si(100) substrates was characterized by Raman spectroscopy [
211]. The influence of the deposition temperature on the microstructure was investigated by an examination of the rigid layer-like mode at 145 cm
−1, which showed a frequency shift with increasing
Ts. The ability of the V
2O
5 thin film lattice to accommodate Li
+ ions was also investigated by Raman spectroscopy. The appearance of the δ- and γ-phases of Li
xV
2O
5 gave additional insight into the structural changes of lithiated films. From the photoluminescence spectra, Iida et al. evidenced a blue shift of the vanadyl V = O peak upon Li
+ insertion for different electric charge in the range of 0 ≤
Q ≤ 20 mC of Li
+ [
212]. From X-ray diffraction and Raman spectroscopy data, Shibuya et al. derived a
PO₂ –
Ts phase diagram for V-O films grown on Si(100) substrates (
Figure 12). The composition of V-O films was as follows: (i) A VO
2 monoclinic phase was formed at
Ts ≥ 450 °C and
PO2 in the range of 5 to 20 mTorr; (ii) a V
2O
5 orthorhombic phase was obtained under oxidative conditions, i.e., at high
PO₂; (iii) a V
6O
13 phase was grown under
PO₂ between oxidative and reductive conditions; and (iv) metastable V
4O
9 and VO
2(B) phases were formed for lower
Ts (≤400 °C) and lower
PO₂ (≤30 mTorr) [
213].
V
2O
5 thin films have been widely used as electrochromic electrodes but few reports are devoted to PLD-grown films. Fang et al. obtained thin films deposited on In
2O
3:SnO
2 (ITO)-coated glass and (111)Si wafer from a V
2O
5 target using an XeCl laser with a wavelength of 308 nm for applications in electrochromic devices [
214,
215]. Electrochromic tests over 60,000 cycles showed that a significant change in the optical density (bleached and colored states) was evaluated to be 0.13 at λ = 600 nm for as-prepared films at
Ts = 200 °C. Crystallized c-axis oriented V
2O
5 films were obtained under oxygen and at a substrate temperature of 200 °C. The durability without long-term degradation of the electrochromic V
2O
5 films was tested over 8000 cycles in the voltage range of 1.2 to 1.4 V [
216]. Ti-doped V
2O
5 thin films prepared by the pulsed laser ablation technique at
Ts = 200 °C and Φ = 2 J·cm
−2 were studied as the electrode for an electrochromic display that exhibits a neutral brownish blue color. The long-term durability was verified over 8000 cycles of a voltage cycled in the range from −1.0 to +1.0 V vs. SCE showing a charge of 35 mC·cm
−2. The good cycleability was attributed to the layered structure of PLD crystalline films with a parallel orientation to the substrate, suitable for Li
+-ions’ transport [
215]. PLD thin films of the system, WO
3-V
2O
5, were prepared with a laser fluence of 1 to 2 J·cm
−2 on SnO
2/F-coated glass substrates at
Ts = 25 °C under
PO₂ = 0.1 mbar. Such films with low V contents cycled in the protonic medium. The true color neutrality is the main advantage of V-based WO
3 thin films; however, the cell capacity and coloration efficiency decrease with an increase of the V content [
217]. The orthorhombic V
2O
5 phase is also applied as electrodes for sensors. Huotari reported that pure PLD films were obtained at Φ = 2.6 J cm
−2,
Ts = 400 °C, and
PO₂ = 1.0 Pa with a post-annealing treatment at 400 °C for 1 h in normal ambient conditions [
218]. The efficient response to NH
3 at part-per-billion levels. indicates these films use as possible sensing materials for ammonia gas [
219].
The electrochemical properties of V
2O
5 thin-film cathode material have been widely studied in cells with aprotic electrolytes (typically LiClO
4 dissolved in propylene carbonate). The electrochemical charge–discharge profiles of PLD V
2O
5 films were also found to be dependent on
Ts, exhibiting a marked difference for V
2O
5 films grown at
Ts < 200 °C when compared to those grown at
Ts ≥ 200 °C. The effect of the substrate temperature and hence the microstructure on the kinetics of the lithium intercalation process in V
2O
5 films is remarkable. The applicability of the grown PLD V
2O
5 films in lithium microbatteries indicates that PLD V
2O
5 films in the temperature range of 200 to 400 °C offer better electrochemical performance than films grown at other temperatures due to their excellent structural quality and stability [
25,
220]. As an experimental fact, pulsed laser deposited V
2O
5 thin films exhibit a higher initial voltage than the crystalline material, i.e., ~4.1 vs. ~3.5 V (Li
+/Li). For instance, V
2O
5 thin-film cathodes, deposited from a V
6O
13 target at a fluence of ~12 J cm
−2 on SnO
2-coated glass at
Ts = 200 °C, were efficient for Li
+-ion incorporation. In (
h00)-textured films, the specific capacity reached values between 50% and 80% of the theoretical value. On the other hand, amorphous films display a stable capacity corresponding to 1.2 F mol
−1 in the voltage range of 4.1 to 1.5 V. Prior textured V
2O
5 films discharged beyond the threshold to 2.0 V vs. Li
+/Li showed an immediate and continuous capacity fading and a quasi-total amorphization after 10 cycles [
193,
197]. The chemical diffusion coefficient of Li
+ ions,
D*, measured by PITT was found to be in the range of 1.7 × 10
−12 to 5.8 × 10
−15 cm
2·s
−1 in crystalline V
2O
5 films, which compares well to the value found in Li
xV
2O
5 phases, whereas
D* displayed a smooth and continuous decrease as the Li content increased in amorphous films [
198].
In an attempt to apply PLD V
2O
5 films in SSMB, a thin-film microbattery was constructed using a glassy Li
1.4B
2.5S
0.1O
4.9 electrolyte film with an ionic conductivity of 5 × 10
−6 S·cm
−1 at 25 °C and an Li anode film. This Li/Li
1.4B
2.5S
0.1O
4.9/V
2O
5 cell delivered a capacity of ~400 mC·cm
−2·µm
−1 at a current density of 15 µA·cm
−2 [
221]. Ag
0.3V
2O
5 and LiPON thin films with a smooth surface were grown by PLD in an N
2 and O
2 atmosphere, respectively. The Li/LiPON/Ag
0.3V
2O
5 SSMB displayed good cycleability at a current density of 7 µA·cm
−2 in the voltage window of 1.0 to 3.5 V. The specific capacity was maintained at 40 µAh·cm
−2·µm
−1 after 100 cycles [
222]. Recently, amorphous vanadium oxide a-VO
x PLD films (650 nm thick) were grown on stainless steel substrates from a V
2O
5 PLD-target under
PO₂ in the range of 0 to 30 Pa. Films prepared under
PO₂ = 13 Pa had a smooth surface and bore an O/V atomic ratio of 2.13 with a higher atomic percentage of V
5+ than that of V
4+. Electrochemical tests carried out in Li cells with 1 mol·L
−1 LiPF
6 in ethylene carbonate (EC) and diethyl carbonate (DEC) (1:1 by volume) as the electrolyte showed a reversible specific capacity as high as 300 mAh·g
−1 at the C/10 current rate and a capacity retention of 90% after 100 cycles [
223]. Such studies were initiated by Zhang et al. in 1997 to obtain VO
x films PLD grown at 200 °C and exhibiting a specific capacity of 340 mAh·g
−1 at a current density of 0.1 mA·cm
−2 and a capacity loss <2% at the end of 100 cycles [
201]. A summary of the electrochemical properties of PLD-grown vanadium oxide thin film electrodes is given in
Table 7.
3.16. MoO3
MoO
3 is an attractive cathode material for microbattery technology from several standpoints: (i) The orthorhombic a-phase is a layered structure favorable for Li insertion between slabs; (ii) Mo has the highest +6 oxidation state, making the high structural stability; (iii) the lattice can be reversibly inserted up to 1.5Li per mole of oxide, yielding a specific capacity of 280 mAh·g
−1; and (iv) the capacity of the dense film can reach a value of ≈130 µAh·cm
−2·µm
−1, almost twice the value for LiCoO
2 [
229]. In addition to the use as cathode batteries, MoO
3 is a material applied in electrochromics, gas sensors, and electro-optics. For certain applications, high-quality films grown by PLD are required.
Currently, PLD MoO
3 thin films are grown using a KrF excimer laser (λ = 248 nm) with a fluence of 2 J cm
−2 (energy of 300 mJ per pulse) and deposited on various substrates heated in the range of 25 ≤
Ts ≤ 500 °C under an atmosphere of O
2 flow maintained at a pressure of 0.1 ≤
PO₂ ≤ 20 Pa. In the prior report, Julien et al. showed that the structure analyzed by optical spectroscopy strongly depends on
Ts: For
Ts < 150 °C, an amorphous phase is formed, the β-MoO
3 phase grows at
Ts ≈ 200 °C, and the layered α-MoO
3 phase appears at
Ts = 300 °C [
230,
231,
232,
233]. Al-Kuhaili et al. reported the growth of polycrystalline MoO
3 films on unheated substrates using both XeF and KrF excimer lasers. By tuning the annealing temperature in the range of 300 to 500 °C, both the grain size and surface roughness increased. Films formed using the XeF laser (λ = 351 nm) and annealed at 400 °C have the best stoichiometry of MoO
2.95 [
233]. Analyzing the growth mechanism, Ramana and Julien concluded that the thermochemical reaction during ablation strongly influences the structural characteristics of PLD MoO
3 films. Above
Ts = 400 °C, the formation of compositional defects induces structural disorder, i.e., α-β-MoO
3−x phase mixture [
234,
235].
The applicability of PLD films to an Li microbattery was demonstrated by the best electrochemical features: A discharge capacity of 90 µAh cm
−2 µm
−1 was obtained for
Ts = 400 °C, while only 53 µAh·cm
−2·µm
−1 was delivered for
Ts = 200 °C [
236]. Puppala et al. investigated the microstructure and morphology of PLD MoO
3−x thin films’ growth for catalytic applications using a femtosecond laser (f-PLD) and a nanosecond excimer-laser (n-PLD). Substantially textured films with a partially crystalline phase prior to annealing were obtained by the f-PDL laser, while the n-PLD-grown MoO
3−x films were predominantly amorphous with a smooth surface [
237]. Sunu et al. claimed that as-deposited PLD films (
Ts = 400 °C, Φ = 4–5 J·cm
−2, repetition rate of 15 to 20 Hz, and
PO₂ = 500 Pa) are suboxide-like, i.e., mixture of η-Mo
4O
11 and χ-Mo
4O
11, which transformed to MoO
3 after annealing at 500 °C in air for 5 h [
238]. Several works reported the PLD growth of films (MoO
3)
1−x(V
2O
5)
x with 0.0 ≤
x ≤ 0.3 prepared at room temperature under an oxygen pressure of 13.3 Pa. The effect of the V
2O
5 content on the coloring switching properties for thermochromic, gasochromic, photochromic, and electrochromic applications was investigated [
239,
240]. Contrary to pure MoO
3, the electrochromism of MoO
3-V
2O
5 films showed that the Mo oxidation state (+6) did not change considerably upon Li
+ insertion, while V
5+ was reduced considerably to V
4+ [
239]. A similar improvement of the gas-sensing properties, i.e., the shortest response time and highest transmittance change, was observed for V
2O
5-doped MoO
3 films under an H
2 atmosphere [
240].
3.17. WO3
Tungsten oxide (WO
3) belongs to the class of “chromogenic” materials, i.e., materials exhibiting coloration effects through electro-, photo-, gas-, laser-, and thermochromism processes, which requires the high homogeneity provided by the PLD technique. Preliminary studies of the growth of WO
3 thin films by PLD were first attempted by Haro-Poniaowski et al. [
233] in 1998. Later, Rougier et al. reported the PLD conditions for the growth of efficient WO
3 films as electrochromics (EC) components [
241]. The microstructure of films deposited on SnO
2:F coated glass substrate is strongly sensitive to both the oxygen pressure and substrate temperature: (i) Crystallized films are formed for
Ts = 400 °C and
PO₂ = 10 Pa; (ii) amorphous films are obtained for
PO₂ = 1 Pa at any
Ts; (iii) for
Ts = 25 °C and
PO2 = 1 Pa, WO
3 films are blue colored and conductive; and (iv) colorless insulator films are grown for
Ts = 25 °C and
PO₂ = 10 Pa, which display the best electrochromic properties. Qiu and Lu showed that oxygen deficient WO
3−δ films with a deviated monoclinic structure were produced using PLD parameters as 2.5 J·cm
−2,
PO₂ = 26 Pa, and a target-Si(100) substrate distance of
d = 5 cm [
242]. Ramana et al. investigated the structural transformations of PLD WO
3 as a function of the annealing treatment. Using standard conditions (Φ = 2 J·cm
−2,
Ts = 300 °C,
PO₂ = 13.3 Pa), films deposited on glass substrates (200–500 nm thick) showed an atomic ratio of O/W ≈ 2.96 ± 0.05. The monoclinic phase of the as-prepared film transformed to an orthorhombic phase at 350 °C and to a hexagonal phase at 500 °C [
243,
244]. By varying the substrate temperature in the range of 150 to 800 °C and the oxygen pressure from 1 to 40 Pa, Mitsugi et al. obtained WO
3 films with a different microstructure: Amorphous, crystallized tetragonal, and triclinic phases [
245]. Hussain et al. obtained amorphous, polycrystalline, and nanocrystalline WO
3 phases, and iso-epitaxial WO
3(00
l) thin films deposited on single-crystal SrTiO
3 substrates at 600 °C and under
PO₂ = 18 Pa [
246]. Suda et al. deposited PLD WO
3 thin films on flexible ITO substrates. They showed that films, prepared at
Ts < 300 °C, are amorphous and polycrystalline phases were obtained at
Ts > 400 °C, while the crystallinity of the film on glass substrates was not dependent on
PO₂ [
247]. Films deposited at 400 °C were porous with a nanocrystalline triclinic structure and showed the best cycleability [
216,
248,
249].
The suitability of PLF WO
3 films for EC applications was investigated as a function of the partial oxygen pressure during deposition. Studies of the texture and morphology of PLD 30-nm thick WO
3 films deposited on Si(100) and SrTiO
3(100) substrates under an O
2 background of 2.5 Pa showed that: (i) The laser fluence (in the range of 5 to 15 J·cm
−2) strongly influences the texture, (ii) the films grown on STO are biaxially textured with a smooth surface, and (iii) films deposited on Si are granular [
250]. The fabrication of WO
3 thin films with color neutrality for applications as EC materials was realized by the deposition of films containing 20% of vanadium onto SnO
2:F coated glasses at
Ts = 20 °C under
PO₂ = 10 Pa. The blue color in the reduced state (−0.4 V) of the W-O-V films lost intensity and turned grey-blue (transmittance of 50%) as the V concentration increased [
251]. Highly transparent WO
3 films exhibiting strong coloration and fast and full bleaching were prepared under PLD conditions (Φ = 1 J·cm
−2,
Ts = 250 °C,
PO₂ = 16 Pa, and
d = 40 mm) [
252]. WO
3 films were also prepared using similar PLD parameters for applications in gas sensors [
253,
254,
255].