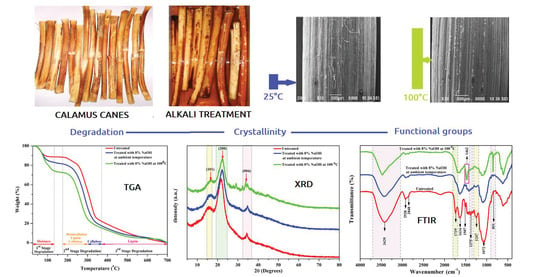
Effect of Alkali Treatment under Ambient and Heated Conditions on the Physicochemical, Structural, Morphological, and Thermal Properties of Calamus tenuis Cane Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alkali Treatment of CTCFs
2.3. Characterization of Untreated and Treated CTCFs
2.3.1. Measurement of Bulk Density and Diameter of Calamus tenuis Canes
2.3.2. Chemical Analysis
2.3.3. Fourier Transformed Infrared (FTIR) Analysis
2.3.4. X-ray Diffraction (XRD) Analysis
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Scanning Electron Microscope (SEM) Analysis
3. Results
3.1. Density and Diameter of Untreated and Treated Calamus tenuis Canes
3.2. Chemical Analysis
3.3. Fourier Transform Infrared (FTIR) Analysis
3.4. X-ray Diffraction (XRD) Analysis
3.5. Thermogravimetric Analysis (TGA)
3.6. Scanning Electron Microscope (SEM) Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santulli, C.; Fragassa, C.; Pavlovic, A.; Nikolic, D. Use of Sea Waste to Enhance Sustainability in Composite Materials: A Review. J. Marine Sci. Eng. 2023, 11, 855. [Google Scholar] [CrossRef]
- Saikia, D. Studies of Water Absorption Behavior of Plant Fibers at Different Temperatures. Int. J. Thermophys. 2010, 31, 1020–1026. [Google Scholar] [CrossRef]
- Saikia, D. Investigations on Structural Characteristics; Thermal Stability; and Hygroscopicity of Sisal Fibers at Elevated Temperatures. Int. J. Thermophys. 2008, 29, 2215–2225. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, C.; Wang, A.; Wang, Y.; Xu, W. Morphologies and properties of Juncus effusus fiber after alkali treatment. Cellulose 2020, 27, 1909–1920. [Google Scholar] [CrossRef]
- Balaji, A.N.; Nagarajan, K.J. Characterization of alkali treated and untreated new cellulosic fiber from Saharan aloe vera cactus leaves. Carbohydr. Polym. 2017, 174, 200–208. [Google Scholar]
- Shanmugasundaram, N.; Rajendran, I.; Ramkumar, T. Characterization of untreated and alkali treated new cellulosic fiber from an Areca palm leaf stalk as potential reinforcement in polymer composites. Carbohydr. Polym. 2018, 195, 566–575. [Google Scholar]
- Shahril, S.M.; Ridzuan, M.J.M.; Majid, M.S.A.; Bariah, A.M.N.; Rahman, M.T.A.; Narayanasamy, P. Alkali treatment influence on cellulosic fiber from Furcraea foetida leaves as potential reinforcement of polymeric composites. J. Mater. Res. Technol. 2022, 19, 2567–2583. [Google Scholar] [CrossRef]
- Arul Marcel Moshi, A.; Ravindran, D.; Sundara Bharathi, S.R.; Indran, S.; Suganya Priyadharshini, G. Characterization of surface-modified natural cellulosic fiber extracted from the root of Ficus religiosa tree. Int. J. Biol. Macromol. 2020, 156, 997–1006. [Google Scholar] [CrossRef]
- Tenazoa, C.; Savastano, H.; Charca, S.; Quintana, M.; Flores, E. The Effect of Alkali Treatment on Chemical and Physical Properties of Ichu and Cabuya Fibers. J. Nat. Fibers 2021, 18, 923–936. [Google Scholar] [CrossRef]
- Saikia, D. Studies on Thermo-Physical Properties of Some Textile Fibres (Plant). Ph.D. Thesis, Gauhati University, Guwahati, India, 2003. Available online: https://ui.adsabs.harvard.edu (accessed on 13 October 2023).
- Saikia, D.; Predeep, P.; Prasanth, S.; Prasad, A.S. The Effect of Heat on Structural Characteristics and Water Absorption Behavior of Agave Fibers. AIP Conf. Proc. 2008, 1004, 48–52. [Google Scholar]
- Umashankaran, M.; Gopalakrishnan, S. Effect of Sodium Hydroxide Treatment on Physico-chemical; Thermal; Tensile and Surface Morphological Properties of Pongamia pinnata L. Bark Fiber. J. Nat. Fibers 2021, 18, 2063–2076. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-food wastes for bioplastics: European prospective on possible applications in their second life for a circular economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef] [PubMed]
- Hamid, L.; Elhady, S.; Abdelkareem, A.; Fahim, I. Fabricating starch-based bioplastic reinforced with bagasse for food packaging. Circ. Econ. Sust. 2022, 2, 1065–1076. [Google Scholar] [CrossRef]
- Navarro Gausa, M.; Pericu, S.; Canessa, N.; Tucci, G. Creative Food Cycles: A Cultural Approach to the Food Life-Cycles in Cities. Sustainability 2020, 12, 6487. [Google Scholar] [CrossRef]
- Saikia, D.; Pratap, A.; Saxena, N.S. Moisture Absorption of Plant Fiber under Annealed; Bleached and Mercerized Condition. AIP Conf. Proc. 2010, 1249, 95–98. [Google Scholar] [CrossRef]
- Baskaran, P.G.; Kathiresan, M.; Pandiarajan, P. Effect of Alkali-treatment on Structural; Thermal; Tensile Properties of Dichrostachys cinerea Bark Fiber and Its Composites. J. Nat. Fibers 2022, 19, 433–449. [Google Scholar] [CrossRef]
- Bezazi, A.; Boumediri, H.; Garcia del Pino, G.; Bezzazi, B.; Scarpa, F.; Reis, P.N.B. Alkali Treatment Effect on Physicochemical and Tensile Properties of Date Palm Rachis Fibers. J. Nat. Fibers 2022, 19, 3770–3787. [Google Scholar] [CrossRef]
- Palanisamy, S.; Mayandi, K.; Dharmalingam, S.; Rajini, N.; Santulli, C.; Mohammad, F.; Al-Lohedan, H.A. Tensile Properties and Fracture Morphology of Acacia caesia Bark Fibers Treated with Different Alkali Concentrations. J. Nat. Fibers 2022, 19, 11258–11269. [Google Scholar] [CrossRef]
- Geethamma, V.G.; Joseph, R.; Thomas, S. Short coir fiber-reinforced natural rubber composites: Effects of fiber length, orientation, and alkali treatment. J. Appl. Polym. Sci. 1995, 55, 583–594. [Google Scholar] [CrossRef]
- Vijay, R.; Vinod, A.; Lenin Singaravelu, D.; Sanjay, M.R.; Siengchi, S. Characterization of chemical treated and untreated natural fibers from Pennisetum orientale grass—A potential reinforcement for lightweight polymeric applications. Int. J. Lightweight Mater. Manuf. 2021, 4, 43–49. [Google Scholar] [CrossRef]
- Kar, A.; Saikia, D. Characterization of new natural cellulosic fiber from Calamus tenuis (Jati Bet) cane as a potential reinforcement for polymer composites. Heliyon 2023, 9, 16491. [Google Scholar] [CrossRef]
- Rony, R. Phytochemical screening, antioxidant and cytotoxic activity of fruit extracts of Calamus tenuis Roxb. J. Coast. Life Med. 2014, 2, 645–650. [Google Scholar]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Sudhakar, P.; Baskaran, R. Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark. Carbohydr. Polym. 2013, 92, 1928–1933. [Google Scholar] [CrossRef]
- Jamilah, U.L.; Sujito, S. The Improvement of Ramie Fiber Properties as Composite Materials Using Alkalization Treatment, NaOH Concentration. J. Sains Mater. Indones. 2021, 22, 62–70. [Google Scholar] [CrossRef]
- Herlina Sari, N.; Wardana, I.N.G.; Irawan, Y.S.; Siswanto, E. Characterization of the Chemical; Physical; and Mechanical Properties of NaOH-treated Natural Cellulosic Fibers from Corn Husks. J. Nat. Fibers 2018, 15, 545–558. [Google Scholar] [CrossRef]
- Jang, E.S.; Kang, C.W. Changes in gas permeability and pore structure of wood under heat treating temperature conditions. J. Wood Sci. 2019, 65, 37. [Google Scholar] [CrossRef]
- Dampanaboina, L.; Yuan, N.; Mendu, V. Estimation of Crystalline Cellulose Content of Plant Biomass using the Updegraff Method. J. Vis. Exp. 2021, 171, e62031. [Google Scholar] [CrossRef]
- Agu, C.V.; Njoku, O.U.; Chilaka, F.C.; Agbiogwu, D.; Iloabuchi, K.V.; Ukazu, B. Physicochemical properties of lignocellulosic biofibres from South Eastern Nigeria, Their suitability for biocomposite technology. Afr. J. Biotechnol. 2014, 13, 2050–2057. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Farrukh, M.A.; Butt, K.M.; Chon, K.-K.; Chang, W.S. Photoluminescence emission behavior on the reduced band gap of Fe doping in CeO2-SiO2 nanocomposite and photophysical properties. J. Saudi Chem. Soc. 2019, 23, 561–575. [Google Scholar] [CrossRef]
- Talavera-Pech, W.A.; Montiel-Rodríguez, D.; Paat-Estrella, J.D.L.A.; López-Alcántara, R.; Pérez-Quiroz, J.T.; Pérez-López, T. Improvement in the carbonation resistance of construction mortar with cane bagasse fiber added. Materials 2021, 14, 2066. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Almeida, J.S.; Dias, A.F.; Figueirêdo, M.C.B.; Morais, J.P.S.; Feitosa, J.P.; Rosa, M.D.F. A novel green approach for the preparation of cellulose nanowhiskers from white coir. Carbohydr. Polym. 2014, 110, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Li, N.; Zong, M.H. Facile and simple pretreatment of sugar cane bagasse without size reduction using renewable ionic liquids–water mixtures. ACS Sustain. Chem. Eng. 2013, 1, 519–526. [Google Scholar] [CrossRef]
- Batalha, L.A.R.; Colodette, J.L.; Gomide, J.L.; Barbosa, L.C.; Maltha, C.R.; Gomes, F.J.B. Dissolving pulp production from bamboo. BioResources 2012, 7, 0640–0651. [Google Scholar] [CrossRef]
- Ganapathy, T.; Sathiskumar, R.; Senthamaraikannan, P.; Saravanakumar, S.S.; Khan, A. Characterization of raw and alkali treated new natural cellulosic fibres extracted from the aerial roots of banyan tree. Int. J. Biol. Macromol. 2019, 138, 573–581. [Google Scholar] [CrossRef]
- Babu, B.G.; Princewinston, D.; Saravanakumar, S.; Khan, A.; Bhaskar, P.A.; Indran, S.; Divya, D. Investigation on the Physicochemical and Mechanical Properties of Novel Alkali-treated Phaseolus vulgaris Fibers. J. Nat. Fibers 2022, 19, 770–781. [Google Scholar] [CrossRef]
- Pokhriyal, M.; Rakesh, P.K.; Rangappa, S.M.; Siengchin, S. Effect of alkali treatment on novel natural fiber extracted from Himalayacalamus falconeri culms for polymer composite applications. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Saravanakumar, S.S. Evaluation of characteristic features of untreated and alkali-treated cellulosic plant fibers from Mucuna atropurpurea for polymer composite reinforcement. Biomass Convers. Biorefin. 2023, 13, 11295–11309. [Google Scholar] [CrossRef]
- Loganathan, T.M.; Sultan, M.T.H.; Ahsan, Q.; Jawaid, M.; Naveen, J.; Shah, A.U.; Hua, L.S. Characterization of alkali treated new cellulosic fibre from Cyrtostachys renda. J. Mater. Res. Technol. 2020, 9, 3537–3546. [Google Scholar] [CrossRef]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Characterization of cellulose fibers in Thespesia populnea barks, Influence of alkali treatment. Carbohydr. Polym. 2019, 217, 178–189. [Google Scholar] [CrossRef]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Moorthy, I.G. Effect of Chemical Treatments on Physicochemical Properties of Prosopis juliflora Fibers. Int. J. Polym. Anal. Charact. 2014, 19, 383–390. [Google Scholar] [CrossRef]
- Sivasubramanian, P.; Kalimuthu, M.; Palaniappan, M.; Alavudeen, A.; Rajini, N.; Santulli, C. Effect of alkali treatment on the properties of Acacia caesia bark fibres. Fibers 2021, 9, 49. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Karlsson, S.; Tesha, J.V.; Nyahumwa, C.W. Chemical and physical modifications of rice husks for use as composite panels. Compos. Part. A Appl. Sci. Manuf. 2007, 38, 925–935. [Google Scholar] [CrossRef]
- Selvaraj, M.; Pannirselvam, N.; Ravichandran, P.T.; Mylsamy, B.; Samson, S. Extraction and Characterization of a New Natural Cellulosic Fiber from Bark of Ficus carica Plant as Potential Reinforcement for Polymer Composites. J. Nat. Fibers 2023, 20, 2194699. [Google Scholar] [CrossRef]
- Vârban, R.; Crișan, I.; Vârban, D.; Ona, A.; Olar, L.; Stoie, A.; Ștefan, R. Comparative FT-IR prospecting for cellulose in stems of some fiber plants: Flax, velvet leaf, hemp and jute. Appl. Sci. 2021, 11, 8570. [Google Scholar] [CrossRef]
- Johny, V.; Kuriakose Mani, A.; Palanisamy, S.; Rajan, V.K.; Palaniappan, M.; Santulli, C. Extraction and Physico-Chemical Characterization of Pineapple Crown Leaf Fibers (PCLF). Fibers 2023, 11, 5. [Google Scholar] [CrossRef]
- Ding, L.; Han, X.; Cao, L.; Chen, Y.; Ling, Z.; Han, J.; Jiang, S. Characterization of natural fiber from manau rattan (Calamus manan) as a potential reinforcement for polymer-based composites. J. Biores Bioprod. 2022, 7, 190–200. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Wu, N.; Ma, Y.; Menon, C.; Tong, J. Characterization of natural cellulose fiber from corn stalk waste subjected to different surface treatments. Cellulose 2019, 26, 4707–4719. [Google Scholar] [CrossRef]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the interpretation of X-ray diffraction powder patterns in terms of the nanostructure of cellulose I fibres. Macromol. Chem. Phys. 2005, 206, 1568–1575. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Saravanakumar, S.S.; Sanjay, M.R.; Jawaid, M.; Siengchin, S. Physico-chemical and thermal properties of untreated and treated Acacia planifrons bark fibers for composite reinforcement. Mater. Lett. 2019, 240, 221–224. [Google Scholar] [CrossRef]
- Popescu, C.; Vasile, C.; Popescu, M.; Singurel, G.; Popa, V.I.; Munteanu, B.S. Analytical methods for lignin characterization. II. Spectroscopic studies. Cellul. Chem. Technol. 2006, 40, 597. [Google Scholar]
- Cai, M.; Takagi, H.; Nakagaito, A.N.; Li, Y.; Waterhouse, G.I.N. Effect of alkali treatment on interfacial bonding in abaca fiber-reinforced composites. Compos. Part. A Appl. Sci. Manuf. 2016, 90, 589–597. [Google Scholar] [CrossRef]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Effect of alkali treatment on crystalline structure of cellulose fiber from mendong (Fimbristylis globulosa) straw. Key Eng. Mater. 2014, 594, 720–724. [Google Scholar] [CrossRef]
- Arya, A.; Tomlal, J.E.; Gejo, G.; Kuruvilla, J. Commingled composites of polypropylene/coir-sisal yarn: Effect of chemical treatments on thermal and tensile properties. e-Polymers 2015, 15, 169–177. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Wang, X.; Wang, H.; Wu, Y.; Zhong, T.; Fei, B. Effect of alkali treatment on wettability and thermal stability of individual bamboo fibers. J. Wood Sci. 2018, 64, 398–405. [Google Scholar] [CrossRef]
- Raia, R.Z.; Iwakiri, S.; Trianoski, R.; Andrade, A.S.; Kowalski, E.L. Effects of alkali treatment on modification of the Pinus fibers. Matéria 2021, 26, e12936. [Google Scholar] [CrossRef]
- Ramasamy, R.; Obi Reddy, K.; Varada Rajulu, A. Extraction and Characterization of Calotropis gigantea Bast Fibers as Novel Reinforcement for Composites Materials. J. Nat. Fibers 2018, 15, 527–538. [Google Scholar] [CrossRef]
- Elenga, R.G.; Djemia, P.; Tingaud, D.; Chauveau, T.; Maniongui, J.G.; Dirras, G. Effects of alkali treatment on the microstructure, composition, and properties of the Raffia textilis fiber. Bioresources 2013, 3, 2934–2949. [Google Scholar] [CrossRef]
- Prithiviraj, M.; Muralikannan, R. Investigation of Optimal Alkali-treated Perotis indica Plant Fibers on Physical, Chemical, and Morphological Properties. J. Nat. Fibers 2022, 19, 2730–2743. [Google Scholar] [CrossRef]
- Indran, S.; Raj, R.E. Characterization of new natural cellulosic fiber from Cissus quadrangularis stem. Carbohydr. Polym. 2015, 117, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Fiore, V.; Scalici, T.; Valenza, A. Characterization of a new natural fiber from Arundo donax L. as potential reinforcement of polymer composites. Carbohydr. Polym. 2014, 106, 77–83. [Google Scholar] [CrossRef]
- Oushabi, A.; Sair, S.; Oudrhiri Hassani, F.; Abboud, Y.; Tanane, O.; El Bouari, A. The effect of alkali treatment on mechanical, morphological and thermal properties of date palm fibers (DPFs), Study of the interface of DPF–Polyurethane composite. S. Afr. J. Chem. Eng. 2017, 23, 116–123. [Google Scholar] [CrossRef]
- Yan, L.; Chouw, N.; Yuan, X. Improving the mechanical properties of natural fibre fabric reinforced epoxy composites by alkali treatment. J. Reinf. Plast. Compos. 2012, 31, 425–437. [Google Scholar] [CrossRef]
- Devnani, G.L.; Sinha, S. Extraction; characterization and thermal degradation kinetics with activation energy of untreated and alkali treated Saccharum spontaneum (Kans grass) fiber. Compos. B Eng. 2019, 166, 436–445. [Google Scholar] [CrossRef]
- Seki, Y.; Sarikanat, M.; Sever, K.; Durmuşkahya, C. Extraction and properties of Ferula communis (chakshir) fibers as novel reinforcement for composites materials. Compos. B Eng. 2013, 44, 517–523. [Google Scholar] [CrossRef]
- Tamanna, T.A.; Belal, S.A.; Shibly, M.A.H.; Khan, A.N. Characterization of a new natural fiber extracted from Corypha taliera fruit. Sci. Rep. 2021, 11, 7622. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.O.; Reddy, K.R.N.; Zhang, J.; Zhang, J.; Varada Rajulu, A. Effect of Alkali Treatment on the Properties of Century Fiber. J. Nat. Fibers 2013, 10, 282–296. [Google Scholar]





| CTCFs Samples | Physical Properties | Chemical Properties | ||||
|---|---|---|---|---|---|---|
| Bulk Density (kg/m3) | Diameter (mm) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) | |
| Untreated | 526 ± 16 | 8.31 ± 0.48 | 37.43 ± 1.40 | 31.06 ± 1.03 | 28.42 ± 0.81 | 4.11 ± 0.62 |
| Treated at 25 °C | 549 ± 12 | 8.06 ± 0.32 | 51.11 ± 1.62 | 21.57 ± 1.41 | 22.32 ± 1.01 | 4.79 ± 0.32 |
| Treated at 100 °C | 557 ± 18 | 7.56 ± 0.27 | 53.33 ± 0.78 | 19.31 ± 1.12 | 20.81 ± 0.58 | 5.87 ± 0.58 |
| CTCFs Samples | Crystallinity Index (%) | Crystallite Size (nm) | ||||
|---|---|---|---|---|---|---|
| I002 (a.u.) | Iam (a.u.) | CI (%) | 2θ (Degrees) | FWHM (Radians) | CS (nm) | |
| Untreated CTCFs | 5559 | 3481 | 37.38 | 21.67 | 0.05109 | 2.73 |
| Treated at 25° C | 4373 | 2448 | 44.02 | 22.08 | 0.04672 | 2.98 |
| Treated at 100° C | 3664 | 2146 | 41.43 | 22.18 | 0.05024 | 2.78 |
| Fibers | Alkali Treatment | Physical Properties | Chemical Properties | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Density (kg/m3) | Diameter (mm) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) | |||
| Calamus tenuis | Untreated | 526 ± 16 | 8.31 ± 0.48 | 37.4 ± 1.4 | 31.1 ± 1 | 28.4 ± 0.8 | 4.1 ± 0.6 | Here |
| 8% (w/v) (25 °C) | 549 ± 12 | 8.06 ± 0.32 | 51.1 ± 1.6 | 21.6 ± 1.4 | 22.3 ± 1 | 4.8 ± 0.3 | ||
| 8% (w/v) (100 °C) | 557 ± 18 | 7.56 ± 0.27 | 53.3 ± 0.8 | 19.3 ± 1.1 | 20.8 ± 0.6 | 5.9 ± 0.6 | ||
| Ficus religiosa | Untreated | 1246 | 0.0256 | 55.6 | 13.9 | 10.1 | 4.9 | [8] |
| 5% (w/v) | 1272 | 0.0225 | 64.4 | 8.9 | 7.6 | 3.6 | ||
| Pongamia pinnata | Untreated | 1345 | - | 62.3 | 14.6 | 12.5 | 5.5 | [12] |
| 5% (w/v) | 1362 | - | 68.4 | 6.3 | 5.1 | 8.3 | ||
| Phaseolus vulgaris | Untreated | 934 | 0.352 | 62.2 | 7 | 9.1 | - | [37] |
| 5% (w/v) | 963 | 0.345 | 69.5 | 4.3 | 7 | - | ||
| Himalayacalamus falconeri | Untreated | 1300 | 0.104 | 72.5 | 12.7 | 7.8 | - | [38] |
| 5% (w/v) | 1355 | 0.095 | 76.8 | 10.8 | 7.2 | - | ||
| Mucuna atropurpurea | Untreated | 1082 ± 29 | 0.29 ± 0.02 | 58.7 ± 5.7 | 16.3 ± 3.2 | 14.2 ± 3.4 | 8 ± 2.5 | [39] |
| 5% (w/v) | 1136 ± 20 | 0.224 ± 0.014 | 75.2 ± 5.3 | 8 ± 3.1 | 6.7 ± 2.9 | 9.9 ± 2 | ||
| Thespesia populnea | Untreated | 1412 | 0.161 ± 0.039 | 70.3 | 12.6 | 16.3 | 1.8 | [41] |
| 5% (w/v) | 1559 | 0.146 ± 0.090 | 76.4 | 9.6 | 12.8 | 2 | ||
| Perotis indica | Untreated | - | - | 68.4 | 15.7 | 8.4 | 4.3 | [60] |
| 5% (w/v) | - | - | 72.4 | 11.3 | 6.6 | 7.6 | ||
| Fibers | Treatment | Structural Properties | Thermal Properties | Ref. | ||
|---|---|---|---|---|---|---|
| CI (%) | CS (nm) | Thermal Stability (°C) | Max. Degradation Temperature (°C) | |||
| Calamus tenuis cane | Untreated | 37.48 | 2.73 | 217 | 317 | Here |
| 8% (w/v) (25 °C) | 44.02 | 2.98 | 211 | 287 | ||
| 8% (w/v) (100 °C) | 41.43 | 2.78 | 204 | 265 | ||
| Saharan aloe vera cactus leaves | Untreated | 52.6 | 5.6 | 225 | 350 | [5] |
| 5% (w/v) | 56.5 | 5.72 | 231 | 355 | ||
| Furcraea foetida | Untreated | 62.05 | 2.44 | 204 | 357 | [7] |
| 9% (w/v) | 74.35 | 4.15 | 231 | 359 | ||
| Aerial roots of banyan tree | Untreated | 72.47 | 6.28 | 230 | 358 | [36] |
| 5% (w/v) | 76.35 | 7.74 | 230 | 368 | ||
| Himalayacalamus falconeri culms | Untreated | 58.92 | 3.39 | 250 | 356 | [38] |
| 5% (w/v) | 67.79 | 3.8 | 258 | 362 | ||
| Mucuna atropurpurea | Untreated | 24.01 | 2.75 | 200 | 298 | [39] |
| 5% (w/v) | 49.89 | 1.6 | 200 | 320 | ||
| Perotis indica | Untreated | 48.3 | - | - | 330 | [60] |
| 5% (w/v) | 55.43 | - | - | 349 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, A.; Saikia, D.; Palanisamy, S.; Santulli, C.; Fragassa, C.; Thomas, S. Effect of Alkali Treatment under Ambient and Heated Conditions on the Physicochemical, Structural, Morphological, and Thermal Properties of Calamus tenuis Cane Fibers. Fibers 2023, 11, 92. https://0-doi-org.brum.beds.ac.uk/10.3390/fib11110092
Kar A, Saikia D, Palanisamy S, Santulli C, Fragassa C, Thomas S. Effect of Alkali Treatment under Ambient and Heated Conditions on the Physicochemical, Structural, Morphological, and Thermal Properties of Calamus tenuis Cane Fibers. Fibers. 2023; 11(11):92. https://0-doi-org.brum.beds.ac.uk/10.3390/fib11110092
Chicago/Turabian StyleKar, Arup, Dip Saikia, Sivasubramanian Palanisamy, Carlo Santulli, Cristiano Fragassa, and Sabu Thomas. 2023. "Effect of Alkali Treatment under Ambient and Heated Conditions on the Physicochemical, Structural, Morphological, and Thermal Properties of Calamus tenuis Cane Fibers" Fibers 11, no. 11: 92. https://0-doi-org.brum.beds.ac.uk/10.3390/fib11110092