Kinetics of Cellulose Deposition in Developing Cotton Fibers Studied by Thermogravimetric Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
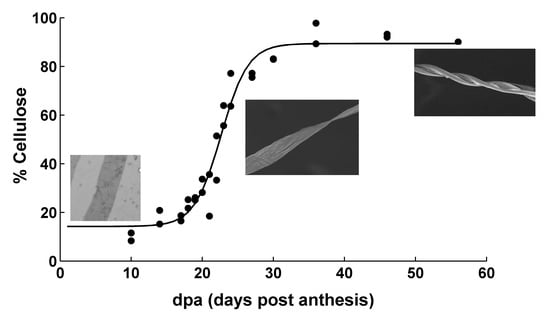
3.1. Cellulose Content
3.2. Thermogravimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Basra, A. Cotton Fibers: Developmental Biology, Quality Improvement, and Textile Processing; Food Products Press; The Haworth Press: Binghamton, NY, USA, 1999. [Google Scholar]
- Hsieh, Y.L. Chapter 6: Structural Development of Cotton Fibers and Linkages to Fiber Quality. In Cotton Fibers: Developmental Biology, Quality Improvement, and Textile Processing; Basra, A., Ed.; Food Products Press; The Haworth Press: Binghamton, NY, USA, 1999; pp. 137–166. [Google Scholar]
- Xu, Y.; Li, H.B.; Zhu, Y.X. Molecular Biological and Biochemical Studies Reveal New Pathways Important for Cotton Fiber Development. J. Integr. Plant Biol. 2007, 49, 69–74. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Cabrales, L.; Gannaway, J.; Wilkins, T.; Wells, L.W. Evaluating cell wall structure and composition of developing cotton fibers using Fourier transform infrared spectroscopy and thermogravimetric analysis. J. Appl. Polym. Sci. 2008, 107, 476–486. [Google Scholar] [CrossRef]
- Haigler, C.H. Physiological and Anatomical Factors Determining Fiber Structure and Utility. In Physiology of Cotton; Stewart, J.M., Oosterhuis, D.M., Heitholt, J.J., Mauney, J.R., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Heyn, A.N.J. The microcrystalline structure of cellulose in cell walls of cotton, ramie, and jute fibers as revealed by negative staining of sections. J. Cell Biol. 1966, 29, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Muhlethaler, K. Ultrastructure and Formation of Plant Cell Walls. Annu. Rev. Plant Physiol. 1967, 18, 1–24. [Google Scholar] [CrossRef]
- Paralikar, K.M. Electron diffraction studies of cotton fibers from bolls during early stages of development. J. Polym. Sci. Part C Polym. Lett. 1986, 24, 419–421. [Google Scholar] [CrossRef]
- Hu, X.P.; Hsieh, Y.L. Crystalline structure of developing cotton fibers. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1451–1459. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Hu, X.P.; Nguyen, A. Strength and Crystalline Structure of Developing Acala Cotton. Text. Res. J. 1997, 67, 529–536. [Google Scholar] [CrossRef]
- Mihranyan, A.; Llagostera, A.P.; Karmhag, R.; Strømme, M.; Ek, R. Moisture sorption by cellulose powders of varying crystallinity. Int. J. Pharm. 2004, 269, 433–442. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, T.; Makarem, M.; Cintrón, M.S.; Cheng, H.N.; Kang, X.; Bacher, M.; Potthast, A.; Rosenau, T.; King, H.; et al. Effects of ball milling on the structure of cotton cellulose. Cellulose 2019, 26, 305–328. [Google Scholar] [CrossRef]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Huwyler, H.R.; Franz, G.; Meier, H. Changes in the composition of cotton fibre cell walls during development. Planta 1979, 146, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Timpa, J.D.; Triplett, B.A. Analysis of cell-wall polymers during cotton fiber development. Planta 1993, 189, 101–108. [Google Scholar] [CrossRef]
- Hoson, T.; Tokumoto, H.; Wakabayashi, K.; Kamisaka, S. Changes in the Sugar Composition and Molecular Mass Distribution of Matrix Polysaccharides during Cotton Fiber Development. Plant Cell Physiol. 2002, 43, 411–418. [Google Scholar]
- Thomasson, J.A.; Manickavasagam, S.; Mengüç, M.P. Cotton fiber quality characterization with light scattering and fourier transform infrared techniques. Appl. Spectrosc. 2009, 63, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Recent Progress in Fourier Transform Infrared (FTIR) Spectroscopy Study of Compositional, Structural and Physical Attributes of Developmental Cotton Fibers. Materials 2013, 6, 299–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidi, N.; Cabrales, L.; Hequet, E. Fourier transform infrared spectroscopic approach to the study of the secondary cell wall development in cotton fiber. Cellulose 2010, 17, 309–320. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Cabrales, L. Changes in sugar composition and cellulose content during the secondary cell wall biogenesis in cotton fibers. Cellulose 2010, 17, 153–160. [Google Scholar] [CrossRef]
- Ball, R.; McIntosh, A.C.; Brindley, J. The role of char-forming processes in the thermal decomposition of cellulose. Phys. Chem. Chem. Phys. 1999, 1, 5035–5043. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N. On the thermal degradation of cellulose in cotton fibers. J. Therm. Anal. Calorim. 2010, 102, 485–491. [Google Scholar] [CrossRef]
- Price, D.M.; Hourston, D.J.; Dumont, F. Thermogravimetry of Polymers. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 8094–8105. [Google Scholar]
- Abidi, N.; Hequet, E.; Ethridge, D. Thermogravimetric analysis of cotton fibers: Relationships with maturity and fineness. J. Appl. Polym. Sci. 2007, 103, 3476–3482. [Google Scholar] [CrossRef]
- Schultz, T.P.; McGinnis, G.D.; Bertran, M.S. Estimation of Cellulose Crystallinity Using Fourier Transform-Infrared Spectroscopy and Dynamic Thermogravimetry. J. Wood Chem. Technol. 1985, 5, 543–557. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Magalhães, W.L.E.; Nisgoski, S.; De Muniz, G.I.B.; Satyanarayana, K.G.; Lazzarotto, M. New and improved method of investigation using thermal tools for characterization of cellulose from eucalypts pulp. Thermochim. Acta 2016, 638, 44–51. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Hequet, E. Thermogravimetric analysis of developing cotton fibers. Thermochim. Acta 2010, 498, 27–32. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Kinetics in Solids. Annu. Rev. Phys. Chem. 1997, 48, 125–149. [Google Scholar] [CrossRef] [PubMed]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Updegraff, D.M. Semimicro determination of cellulose inbiological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Viles, F.J.; Silverman, L. Determination of Starch and Cellulose with Anthrone. Anal. Chem. 1949, 21, 950–953. [Google Scholar] [CrossRef]
- Yin, X.; Goudriaan, J.A.; Lantinga, E.; Vos, J.; Spiertz, H.J. A flexible sigmoid function of determinate growth. Ann. Bot. 2003, 91, 361–371. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, H.; Chen, B.; McGiffen, M.E.; Zhang, W.; Xu, N.; Zhou, Z. The rate of cellulose increase is highly related to cotton fibre strength and is significantly determined by its genetic background and boll period temperature. Plant Growth Regul. 2009, 57, 203–209. [Google Scholar] [CrossRef]
- Abidi, N.; Manike, M. X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Text. Res. J. 2018, 88, 719–730. [Google Scholar] [CrossRef]
- Wang, Z.; McDonald, A.G.; Westerhof, R.J.; Kersten, S.R.; Cuba-Torres, C.M.; Ha, S.; Pecha, B.; Garcia-Perez, M. Effect of cellulose crystallinity on the formation of a liquid intermediate and on product distribution during pyrolysis. J. Anal. Appl. Pyrolysis 2013, 100, 56–66. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Santana, R.M.C.; Zattera, A.J. Materials produced from plant biomass: Part II: Evaluation of crystallinity and degradation kinetics of cellulose. Mater. Res. 2012, 15, 421–427. [Google Scholar] [CrossRef]
- Hideno, A. Comparison of the Thermal Degradation Properties of Crystalline and Amorphous Cellulose, as well as Treated Lignocellulosic Biomass. Bioresources 2016, 11, 6309–6319. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Sengupta, S. Study of the Properties of Microcrystalline Cellulose Particles from Different Renewable Resources by XRD, FTIR, Nanoindentation, TGA and SEM. J. Polym. Environ. 2010, 18, 355–363. [Google Scholar] [CrossRef]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.; Santana, R.M. Thermal decomposition of wood: Influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef] [PubMed]














| Genotype | ||
|---|---|---|
| TM-1 | TX55 | |
| Coefficient a Confidence Interval | [66.67, 83.59] | [68.94, 100.75] |
| Coefficient b Confidence Interval | [21.99, 23.33] | [19.30, 21.77] |
| Coefficient c Confidence Interval | [1.22, 2.55] | [1.47, 3.70] |
| Coefficient d Confidence Interval | [7.99, 20.44] | [−3.07, 23.30] |
| Coefficients | |||||
|---|---|---|---|---|---|
| Graph | a | b | c | d | R2 |
| Cellulose % TM-1 (Figure 1) | 75.17 | 22.66 | 1.89 | 14.22 | 0.91 |
| Crystallinity TX55 (Figure 4) | −2.67 | 19.99 | −1.78 | 4.71 | 0.95 |
| WL 37–150 °C TM-1 (Figure 6) | 4.31 | 23.04 | −2.07 | 5.65 | 0.89 |
| WL 150–400 °C TM-1 (Figure 9) | 25.43 | 20.96 | 1.4 | 51.27 | 0.94 |
| Peak 300–400 °C TM-1 (Figure 12) | 15.55 (14.17 *) | 22.13 (21.92 *) | 0.76 (0.62 *) | 368.94 (368.99 *) | 0.82 (0.87 *) |
| Remaining % TM-1 (Figure 14) | 13.15 | 20.72 | −1.58 | 13.69 | 0.84 |
| Cellulose % TX55 (Figure S2) | 84.85 | 20.53 | 2.59 | 10.66 | 0.95 |
| Crystallinity TM-1 (Figure S3) | −2.51 | 22.42 | −0.75 | 4.67 | 0.97 |
| WL 37–150 °C TX55 (Figure S4) | 4.48 | 20.47 | −1.53 | 5.15 | 0.91 |
| WL 150–400 °C TX55 (Figure S6) | 26.02 | 18.81 | 1.51 | 49.03 | 0.79 |
| Peak 300–400 °C TX55 (Figure S8) | 14.03 (12.85 *) | 19.25 (19.30 *) | 1.73 (1.26 *) | 367.03 (367.66 *) | 0.67 (0.76 *) |
| Remaining % TX55 (Figure S10) | 12.69 | 18.62 | −1.57 | 16.05 | 0.53 |
| Graph | Coefficient b Confidence Interval |
|---|---|
| Cellulose % TM-1 (Figure 1) | [21.59, 24.50] |
| Crystallinity TX55 (Figure 4) | [19.08, 20.91] |
| WL 37–150 °C TM-1 (Figure 6) | [21.59, 24.50] |
| WL 150–400 °C TM-1 (Figure 9) | [20.39, 21.54] |
| Peak 300–400 °C TM-1 (Figure 12) | [21.36, 22.90] [21.34, 22.50] * |
| Remaining % TM-1 (Figure 14) | [19.58, 21.87] |
| Cellulose % TX55 (Figure S2) | [19.30, 21.77] |
| Crystallinity TM-1 (Figure S3) | [22.03, 22.81] |
| WL 37–150 °C TX55 (Figure S4) | [19.67, 21.27] |
| WL 150–400 °C TX55 (Figure S6) | [17.54, 20.08] |
| Peak 300–400 °C TX55 (Figure S8) | [17.15, 21.34] [17.65, 20.95] * |
| Remaining % TX55 (Figure S10) | [16.34, 20.91] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrales, L.; Abidi, N. Kinetics of Cellulose Deposition in Developing Cotton Fibers Studied by Thermogravimetric Analysis. Fibers 2019, 7, 78. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7090078
Cabrales L, Abidi N. Kinetics of Cellulose Deposition in Developing Cotton Fibers Studied by Thermogravimetric Analysis. Fibers. 2019; 7(9):78. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7090078
Chicago/Turabian StyleCabrales, Luis, and Noureddine Abidi. 2019. "Kinetics of Cellulose Deposition in Developing Cotton Fibers Studied by Thermogravimetric Analysis" Fibers 7, no. 9: 78. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7090078