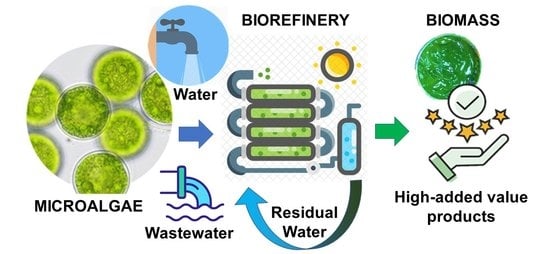
Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Photoautotrophic Production of High-Added Value Products
3. Mixotrophic Production of High-Added Value and Low-Value Products Using Wastewater
4. Production of Microalgae Biomass Enriched in Carbohydrates
4.1. Environmental and Nutritional Factors Affecting Microalgae Cultures
4.2. The Effect of Irradiance on Carbohydrate Accumulation
4.3. Nutritional Factors (N, P and S) That Affect Carbohydrate Accumulation
4.3.1. Nitrogen
4.3.2. Phosphorus
4.3.3. Sulfur
4.4. Strategies to Stimulate Carbohydrate Concentration
4.4.1. Selection of the Appropriate Microalgae
- Tolerance to acute stress (especially in closed photobioreactors);
- To become dominant in relation to contaminating microorganisms;
- High CO2 absorption capacity in photoautotrophic systems (high photosynthetic efficiency);
- Tolerance to large temperature variations resulting from daily and seasonal cycles;
- Low nutrients requirement;
- Potential to obtain high value-added co-products added to the desired products.
- Presence of short productive cycles;
- To show auto flocculation, useful for the microalgal biomass recovery stage.
4.4.2. Operating Modes and Cultivation Systems
4.4.3. Use of Wastewater for Microalgae Cultivation
4.5. Production of Bioethanol from Microalgal Biomass
5. Polysaccharides from Cyanobacteria for Developing New Bioactive Products
- (i)
- Most of the polymers so far studied (up to now close to 200) are characterized by the presence in the macromolecule of a large number of different types of monosaccharides (in about 75% of the cases ranging from 6 up to 15), a feature rarely found in the EPS of other microorganisms. This feature makes it possible to have polymers with similar composition but organized in various structures and conformational forms, enlarging the chances of finding new products with useful properties;
- (ii)
- In most of the polymers are present uronic acids and sulphate groups, which are rare in other bacterial EPS and confer an anionic charge to the polymers. In addition to that, the presence of sulphate groups has been considered relevant for conferring antiviral properties to the polymers [141];
- (iii)
- The presence of peptides and deoxysugars, which confer hydrophobicity of some areas of the polymers;
- (iv)
- The frequent presence of uncommon sugars, such as acetylated or amine sugars, which are considered possibly involved in polymers’ biological activity.
6. Microalgae-Bioactive Compounds, A Natural Source of Potential Therapeutical and Health Promoting Agents: Insights from Innovative In Vivo Functional Studies
Algal Health-Promoting Agents
- Potential skin healing/antifibrotic agents:
- Promising liver health-promoters:
- Natural immunostimulators:
- Non-toxic anticancer agents:
- Improvement of nutrients deficiency:
- Prevention of type 2 Diabetes Mellitus development:
- Natural antioxidant agents:
7. Other Promising/Novel Trends
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food. Sci. Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Olguín, E.J.; Diels, L.; De Philippis, R. Microbial fixation of CO2 in water bodies and in drylands to combat climate change, soil loss and desertification. New Biotechnol. 2015, 32, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Kondo, A.; Hasunuma, T.; Chang, J.S. Engineering strategies for improving the CO fixation and carbohydrate productivity of Scenedesmus obliquus CNW-N used for bioethanol fermentation. Bioresour. Technol. 2013, 143, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Wang, Z.M. Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer Nature: Singapore, 2019; p. 655. [Google Scholar] [CrossRef]
- Patel, A.K.; Choi, Y.Y.; Sim, S.J. Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Xu, J.L.; Wang, Z. Microalgae Biotechnology for Food, Health and High Value Products; Springer Nature: Singapore, 2020; p. 483. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Energy from microalgae: A short history. In Algae for Biofuels and Energy. Developments in Applied Phycology; Borowitzka, M., Moheimani, N., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2013; Volume 5, pp. 1–15. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Dourou, M.; Dritsas, P.; Baeshen, M.N.; Elazzazy, A.; Al-Farga, A.; Aggelis, G. High-added value products from microalgae and prospects of aquaculture wastewaters as microalgae growth media. FEMS Microbiol. Lett. 2020, 367, fnaa081. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Vaidyanathan, S. Microalgae: A robust “green bio-bridge” between energy and environment. Crit. Rev. Biotechnol. 2018, 38, 351–368. [Google Scholar] [CrossRef] [Green Version]
- International Energy Agency (IEA). IEA BIOENERGY Task42 BIOREFINING. In Sustainable and Synergetic Processing of Biomass into Marketable Food & Feed Ingredients, Chemicals, Materials and Energy; van Ree, R., van Zeeland, A., Eds.; IEA: Wageningen, The Netherlands, 2014; Available online: http://www.ieabioenergy.com/wp-content/uploads/2014/09/IEA-Bioenergy-Task42-Biorefining-Brochure-SEP2014_LR.pdf (accessed on 3 May 2022).
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2005, 64, 137–145. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Martinez-Hernandez, E.; Amezcua-Allieri, M.A.; Aburto, J.; Honorato, J.A. Economic and environmental impact evaluation of various biomass feedstock for bioethanol production and correlations to lignocellulosic composition. Bioresour. Technol. Rep. 2019, 7, 100230. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Campbell, G.; Sadhukhan, J. Economic value and environmental impact (EVEI) analysis of biorefinery systems. Chem. Eng. Res. Des. 2013, 91, 1418–1426. [Google Scholar] [CrossRef] [Green Version]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. 3-Microalgae cultivation in wastewater. In Microalgae-Based Biofuels and Bioproducts. From Feedstock Cultivation to End-Products; Woodhead Publishing Series in Energy: Kidlington, UK, 2017; pp. 67–91. [Google Scholar] [CrossRef]
- Olguín, E.J.; Hernández, B.; Araus, A.; Camacho, R.; González, R.; Ramírez, M.E.; Galicia, S.; Mercado, G. Simultaneous high-biomass protein-production and nutrient removal using Spirulina maxima in sea-water supplemented with anaerobic effluents. World J. Microbiol. Biotechnol. 1994, 10, 576–578. [Google Scholar] [CrossRef]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T.J. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Flesch, A.; Beer, T.; Campbell, P.K.; Batten, D.; Grant, T. Greenhouse gas balance and algae-based biodiesel. In Algae for Biofuels and Energy. Developments in Applied Phycology; Borowitzka, M., Moheimani, N., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2013; Volume 5, pp. 233–254. [Google Scholar] [CrossRef]
- Jebali, A.; Acién, F.G.; Barradas, E.R.; Olguín, E.J.; Sayadi, S.; Molina-Grima, E. Pilot-scale outdoor production of Scenedesmus sp. in raceways using flue gases and centrate from anaerobic digestion as the sole culture medium. Bioresour. Technol. 2018, 262, 1–8. [Google Scholar] [CrossRef]
- Li, S.H.; Zhao, S.; Yan, S.L.; Qiu, Y.T.; Song, C.F.; Li, Y.; Kitamura, Y. Food processing wastewater purification by microalgae cultivation associated with high value-added compounds production—A review. Chin. J. Chem. Eng. 2019, 27, 2845–2856. [Google Scholar] [CrossRef]
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef]
- Figueroa-Torres, G.M.; Pittman, J.K.; Theodoropoulos, C. Optimisation of microalgal cultivation via nutrient-enhanced strategies: The biorefinery paradigm. Biotechnol. Biofuels 2021, 14, 64. [Google Scholar] [CrossRef]
- Olguín, E.J. Dual purpose microalgae–bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a biorefinery. Biotechnol. Adv. 2012, 30, 1031–1046. [Google Scholar] [CrossRef]
- Olguín, E.J.; Dorantes, E.; Castillo, O.; Hernández-Landa, V.J. Anaerobic digestates from vinasse promote growth and lipid enrichment in Neochloris oleoabundans cultures. J. Appl. Phycol. 2015, 27, 1813–1822. [Google Scholar] [CrossRef]
- Olguín, E.J.; Castillo, O.S.; Mendoza, A.; Tapia, K.; González-Portela, R.E.; Hernández, V.J. Dual purpose system that treats anaerobic effluents from pig waste and produce Neochloris oleoabundans as lipid rich biomass. New Biotechnol. 2015, 32, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.Y.; Chang, C.K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.S.; Show, P.L. Waste biorefinery towards a sustainable circular bioeconomy: A solution to global issues. Biotechnol. Biofuels 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Calderón, F.V.; López, J.S.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Tasende, M.G.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Herrador, M.; The Microalgae/Biomass Industry in Japan. An Assessment of Cooperation and Business Potential with European Companies. EU-Japan Centre for Industrial Cooperation. Minerva EU-Japan Fellowship. 2016. Available online: https://www.eu-japan.eu/publications/microalgae (accessed on 5 May 2022).
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meticulous Blog. TOP 10 COMPANIES IN SPIRULINA MARKET. Available online: https://meticulousblog.org/top-10-companies-in-spirulina-market/ (accessed on 20 June 2022).
- Meticulous Blog. TOP 10 COMPANIES IN THE CHLORELLA MARKET. Available online: https://meticulousblog.org/top-10-companies-in-the-chlorella-market/ (accessed on 20 June 2022).
- Borowitzka, M.A. High-value products from microalgae-their development and commercialization. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Belay, A. Biology and Industrial Production of Arthrospira (Spirulina). In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; John Wiley & Sons-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 339–358. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Doan, T.T.Y.; Phi, T.C.M.; Ngo, T.A.; Vu, L.D.H.; Dang, D.K. Arthrospira production in Vietnam: Current status and prospects. Bioresour. Technol. Rep. 2021, 15, 100803. [Google Scholar] [CrossRef]
- Meticulous Research. Spirulina Market. Available online: https://www.meticulousresearch.com/product/spirulina-market-5070 (accessed on 19 June 2022).
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction and purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
- Schipper, K.; Fortunati, F.; Oostlander, P.C.; Al Muraikhi, M.; Al Jabri, H.M.S.J.; Wijffels, R.H.; Barbosa, M.J. Production of phycocyanin by Leptolyngbya sp. in desert environments. Algal Res. 2020, 47, 101875. [Google Scholar] [CrossRef]
- García-López, D.A.; Olguín, E.J.; Gonzalez-Portela, R.E.; Sanchez-Galvan, G.; De Philippis, R.; Lovitt, R.W.; Llewellyn, C.A.; Fuentes-Grunewald, C.; Saldivar, R.P. A novel two-phase bioprocess for the production of Arthrospira (Spirulina) maxima LJGR1 at pilot plant scale during different seasons and for phycocyanin induction under controlled conditions. Bioresour. Technol. 2020, 298, 122548. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquac. Int. 2020, 28, 625–638. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, L.; Wei, D. Enhanced production of astaxanthin by Chromochloris zofingiensis in a microplate-based culture system under high light irradiation. Bioresour. Technol. 2017, 245, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Khalili, Z.; Jalili, H.; Noroozi, M.; Amrane, A.; Ashtiani, F.R. Linoleic-acid-enhanced astaxanthin content of Chlorella sorokiniana (Chlorophyta) under normal and light shock conditions. Phycologia 2019, 59, 54–62. [Google Scholar] [CrossRef]
- Kim, Y.E.; Matter, I.A.; Lee, N.; Jung, M.; Lee, Y.C.; Choi, S.A.; Lee, S.Y.; Kim, J.R.; Oh, Y.K. Enhancement of astaxanthin production by Haematococcus pluvialis using magnesium aminoclay nanoplarticles. Bioresour. Technol. 2020, 307, 123270. [Google Scholar] [CrossRef]
- Abiusi, F.; Wijffels, R.H.; Janssen, M. Oxygen balanced mixotrophy under day-night cycles. ACS Sustain. Chem. Eng. 2020, 8, 11682–11691. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, C.H.; Ng, I.S.; Chang, J.S. Enhancing lutein production with mixotrophic cultivation of Chlorella sorokiniana MB-1-M12 using different bioprocess operation strategies. Bioresour. Technol. 2019, 278, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.M.; Sun, Z.; Liu, S.F.; Qin, Z.H.; Mou, J.H.; Zhou, Z.G.; Lin, C.S.K. Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. J. Hazard. Mater. 2020, 400, 123258. [Google Scholar] [CrossRef]
- Lima, S.; Schulze, P.S.C.; Schüler, L.M.; Rautenberger, R.; Morales-Sánchez, D.; Santos, T.F.; Pereira, H.; Varela, J.C.S.; Scargiali, F.; Wijffels, R.H.; et al. Flashing light emitting diodes (LEDs) induce proteins, polyunsaturated fatty acids and pigments in three microalgae. J. Biotechnol. 2021, 325, 15–24. [Google Scholar] [CrossRef]
- Li, D.; Yuan, Y.; Cheng, D.; Zhao, Q. Effect of light quality on growth rate, carbohydrate accumulation, fatty acid profile and lutein biosynthesis of Chlorella sp. AE10. Bioresour. Technol. 2019, 291, 121783. [Google Scholar] [CrossRef]
- Rodrigues, R.D.P.; Silva, A.S.E.; Carlos, T.A.V.; Bastos, A.K.P.; de Santiago-Aguiar, R.S.; Rocha, M.V.P. Application of protic ionic liquids in the microwave-assisted extraction of phycobiliproteins from Arthrospira platensis with antioxidant activity. Sep. Purif. Technol. 2020, 252, 117448. [Google Scholar] [CrossRef]
- Rezvani, S.; Saadaoui, I.; Jabri, H.A.; Moheimani, N.R. Techno-economic modelling of high-value metabolites and secondary products from microalgae cultivated in closed photobioreactors with supplementary lighting. Algal Res. 2022, 65, 102733. [Google Scholar] [CrossRef]
- Montero, E.; Olguín, E.J.; De Philippis, R.; Reverchon, F. Mixotrophic cultivation of Chlorococcum sp. under non-controlled conditions using a digestate from pig manure within a biorefinery. J. Appl. Phycol. 2018, 30, 2847–2857. [Google Scholar] [CrossRef]
- Javed, F.; Aslam, M.; Rashid, N.; Shamir, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chen, C.Y.; Chang, J.-S. Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresour. Technol. 2020, 302, 122817. [Google Scholar] [CrossRef] [PubMed]
- López-Pacheco, I.Y.; Silva-Núñez, A.; García-Pérez, J.S.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Castillo-Zacarías, C.; Afewerki, S.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Phyco-remediation of swine wastewater as a sustainable model based on circular economy. J. Environ. Manag. 2021, 278, 111534. [Google Scholar] [CrossRef]
- Sukumaran, P.; Nulit, R.; Zulkifly, S.; Halimoon, N.; Omar, H.; Ismail, A. Potential of fresh POME as a growth medium in mass production of Arthrospira platensis. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 235–250. Available online: https://www.ijcmas.com/vol-3-4/Puganeswary%20Sukumaran,%20et%20al.pdf (accessed on 9 May 2022).
- Arone Soul Raj, G.P.; Elumalai, S.; Sangeetha, T.; Roop Singh, D. Botryococcus braunii as a phycoremediation tool for the domestic waste water recycling from Cooum River, Chennai, India. J. Bioremed. Biodeg. 2015, 6, 294. [Google Scholar] [CrossRef]
- Basily, H.S.; Nassar, M.M.; El Diwani, G.I.; El-Enin, S.A.A. Exploration of using the algal bioactive compounds for cosmeceuticals and pharmaceutical applications. Egypt. Pharmaceut. J. 2018, 17, 109–120. [Google Scholar] [CrossRef]
- De Francisci, D.; Su, Y.; Iital, A.; Angelidaki, I. Evaluation of microalgae production coupled with wastewater treatment. Environ. Technol. 2018, 39, 581–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safafar, H.; Van Wagenen, J.; Moller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [Green Version]
- Ji, M.K.; Yun, H.S.; Hwang, B.S.; Kabra, A.N.; Jeon, B.H.; Choi, J. Mixotrophic cultivation of Nephroselmis sp. using industrial wastewater for enhanced microalgal biomass production. Ecol. Eng. 2016, 95, 527–533. [Google Scholar] [CrossRef]
- Pan, M.M.; Zhu, X.Y.; Pan, G.; Angelidak, I. Integrated valorization system for simultaneous high strength organic wastewater treatment and astaxanthin production from Haematococcus pluvialis. Bioresour. Technol. 2021, 326, 124761. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Lu, H.; Zhao, Y.; Xu, H.; Zhang, Y.; Li, B. Two-step strategy for obtaining Dunaliella sp. biomass and β-carotene from anaerobically digested poultry litter wastewater. Int. Biodeterior. Biodegrad. 2019, 143, 104714. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Boto-Ordoñez, M.; Van Hulle, S.W.H.; Ferrer, I.; Garfi, M.; Rousseau, D.P.L. Natural pigments from microalgae grown in industrial wastewater. Bioresour. Technol. 2020, 303, 122894. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Veuthey, M.; Morillas-España, A.; Sánchez-Zurano, A.; Navarro-López, E.; Acién, G.; López-Segura, J.G.; Lafarga, T. Production of the marine microalga Nannochloropsis gaditana in pilot-scale thin-layer cascade photobioreactors using fresh pig slurry diluted with sea water. J. Water Process Eng. 2022, 48, 102869. [Google Scholar] [CrossRef]
- Ferreira, A.; Melkonyan, L.; Carapinha, S.; Ribeiro, B.; Figueiredo, D.; Avetisova, G.; Gouveia, L. Bioestimulant and biopesticide potential of microalgae growing in piggery wastewater. Environ. Adv. 2021, 4, 100062. [Google Scholar] [CrossRef]
- Kalra, R.; Gaur, S.; Goel, M. Microalgae bioremediation: A perspective towards wastewater treatment along with industrial carotenoids production. J. Water Process Eng. 2021, 40, 101794. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front Plant Sci. 2018, 7, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acién, F.G.F.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. 2021. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; EL-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Catone, C.M.; Ripa, M.; Geremia, E.; Ulgiati, S. Bio-products from algae-based biorefinery on wastewater: A review. J. Environ. Manag. 2021, 293, 112792. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Alam, M.A.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal and atmospheric carbon mitigation: A review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-cycle análisis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef]
- Debabrata, D. Algal Biorefinery: An Integrated Approach; Springer International Publishing: Cham, Switzerland; Capital Publishing Company: New Delhi, India, 2015; p. 467. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Markham, J.; Templeton, D.W.; Christensen, E.D.; Wychen, S.V.; Vadelius, E.W.; Chen-Glasser, M.; Dong, T.; Davis, R.; Pienkos, P. Development of algae biorefinery concepts for biofuels and bioproducts: A perspective on process-compatible products and their impact on cost-reduction. Energy Environ. Sci. 2017, 10, 1716–1738. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, L.; Graca, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Ferrerira, A.F.; Silva, C.M. Microalgae biomass production using wastewater: Treatment and costs: Scale-up considerations. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Garrido-Cárdenas, J.A.; Manzano-Agugliaro, F.; Acién-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, S.; Liu, C.; Nguyen, L.T.; Hasan, A. The effects of circular economy on economic growth: A quasi-natural experiment in China. J. Clean. Prod. 2020, 271, 122558. [Google Scholar] [CrossRef]
- Acién, F.G.; Gómez-Serrano, C.; Morales-Amaral, M.M.; Fernández-Sevilla, J.M.; Molina-Grima, E. Wastewater treatment using microalgae: How realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bera, S.; Mishra, R.; Roy, S. Redefining the role of microalgae in industrial wastewater remediation. Energy Nexus. 2022, 6, 100088. [Google Scholar] [CrossRef]
- Mishra, S.; Roy, M.; Mohanty, K. Microalgal bioenergy production under zero-waste biorefinery approach: Recent advances and future perspectives. Bioresour. Technol. 2019, 292, 122008. [Google Scholar] [CrossRef]
- Farooq, W. Sustainable production of microalgae biomass for biofuel and chemicals through recycling of water and nutrient within the biorefinery context. A review. GCB Bioenergy 2021, 13, 914–940. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Markou, G.; Nicodemou, A.; Koutinas, M.; Tekerlekopoulou, A.G.; Vayenas, D.V. Cultivation of Arthrospira platensis in Brewery Wastewater. Water 2022, 14, 1547. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol. Adv. 2012, 30, 1655–1661. [Google Scholar] [CrossRef]
- Cheng, D.; Li, D.; Yuan, Y.; Zhou, L.; Li, X.; Wu, T.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving carbohydrate and starch accumulation in Chlorella sp. AE10 by a novel two-stage process with cell dilution. Biotechnol. Biofuels 2017, 10, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 110. [Google Scholar] [CrossRef]
- Bastos, R.G. Biofuels from microalgae: Bioethanol. In Energy from Microalgae. Green Energy and Technology; Jacob-Lopes, E., Queiroz Zepka, L., Queiroz, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 229–246. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Chang, J.S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- da Silva-Braga, V.; da Silveira-Mastrantonio, D.J.; Vieira-Costa, J.A.; Greque de Morais, M. Cultivation strategy to stimulate high carbohydrate content in Spirulina biomass. Bioresour. Technol. 2018, 269, 221–226. [Google Scholar] [CrossRef]
- AST Ingeniería S.L. Oportunidades Empresariales Alrededor de las Microalgas en el Litoral Cantábrico. Aplicaciones de las Microalgas: Estado de la Técnica. [Microalgae Applications: State of the Art] Unión Europea—FSE, AST Ingeniería, S.L. España. 2013. Available online: http://proyectomalgas.com/wp-content/uploads/2014/04/guiamalgas.pdf (accessed on 13 May 2022).
- Daneshvar, E.; Ok, Y.S.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into upstream processing of microalgae: A review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, E.; Filali, R.; Taidi, B. Microalgae culture quality indicators: A review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef]
- Ho, S.S.; Ye, X.; Hasunuma, T.; Chang, J.S.; Kondo, A. Perspectives on engineering strategies for improving biofuel production from microalgae- A review. Biotechnol. Adv. 2014, 32, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; He, Q. Assessment of environmental stresses for enhanced microalgal bioafuel production—An overview. Front. Energy Res. 2014, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Schediwy, K.; Trautmann, A.; Steinweg, C.; Posten, C. Microalgal kinetics- a guideline for photobioreactor design and process development. Eng. Life Sci. 2019, 19, 830–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Miranda, E.; Sánchez-Zurano, A.; Guzmán, J.L.; Acién, G.; Visioli, A. A seasonal simulation approach for culture depth influence on the temperature for different characterized microalgae strains. Biotechnol. J. 2022, 00, e2100489. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Galicia, S.; Angulo-Hernández, O.; Hernández, E. The effect of low light flux and nitrogen deficiency on the chemical composition of Spirulina sp. (Arthrospira) grown on digested pig waste. Bioresour. Technol. 2001, 77, 19–24. [Google Scholar] [CrossRef]
- Morillas-España, A.; Villaró, S.; Ciardi, M.; Acién, G.; Lafarga, T. Production of Scenedesmus almeriensis Using Pilot-Scale Raceway Reactors Located inside a Greenhouse. Phycology 2022, 2, 76–85. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Y.; Xu, H.; Liu, Y.; Sun, J.; Qiao, D.; Cao, Y. Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour. Technol. 2014, 155, 204–212. [Google Scholar] [CrossRef]
- Lisondro, I.; Serrano, C.G.; Sepúlveda, C.; Ceballos, A.I.B.; Acién-Fernández, F.G. Influence of irradiance on the growth and biochemical composition of Nitzschia aff. pellucida. J. Appl. Phycol. 2022, 34, 19–30. [Google Scholar] [CrossRef]
- Dragone, G.; Fernandes, B.D.; Abreu, A.P.; Vicente, A.A.; Teixeira, J.A. Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl. Energy 2011, 88, 3331–3335. [Google Scholar] [CrossRef] [Green Version]
- Ran, W.; Wang, H.; Liu, Y.; Qi, M.; Xiang, Q.; Yao, C.; Zhang, Y.; Lan, X. Storage of starch and lipids in microalgae: Biosynthesis and manipulation by nutrients. Bioresour. Technol. 2019, 291, 121894. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, H.; Li, X.; Qi, W.; Cheng, D.; Tang, T.; Zhao, Q.; Wei, W.; Sun, Y. Enhancing Carbohydrate Productivity of Chlorella sp. AE10 in Semi-continuous Cultivation and Unraveling the Mechanism by Flow Cytometry. Appl. Biochem. Biotechnol. 2018, 185, 419–433. [Google Scholar] [CrossRef]
- Ho, S.H.; Li, P.J.; Liu, C.C.; Chang, J.S. Bioprocess development on microalgae-based CO2 fixation and bioethanol production using Scenedesmus obliquus CNW-N. Bioresour. Technol. 2013, 145, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- Wang, X.; Ruan, Z.; Sheridan, P.; Boileau, D.; Liu, Y.; Liao, W. Two-stage photoautotrophic cultivation to improve carbohydrate production in Chlamydomonas reinhardtii. Biomass Bioenergy 2015, 74, 280–287. [Google Scholar] [CrossRef]
- Tripathi, S.; Choudhary, S.; Poluri, K.M. Insights into lipid accumulation features of Coccomyxa sp. IITRSTKM4 under nutrient limitation regimes. Environ. Technol. Innov. 2021, 24, 101786. [Google Scholar] [CrossRef]
- Yao, C.H.; Ai, J.N.; Cao, X.P.; Xue, S. Characterization of cell growth and starch production in the marine green microalga Tetraselmis subcordiformis under extracellular phosphorus-deprived and sequentially phosphorus-replete conditions. Appl. Microbiol. Biotechnol. 2013, 97, 6099–6110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Z.; Zheng, H.; Wang, Q.; Li, A. Growth, biochemical composition and photosynthetic performance of Scenedesmus acuminatus under different initial sulfur supplies. Algal Res. 2020, 45, 101728. [Google Scholar] [CrossRef]
- Wang, Y.; Chiu, S.Y.; Ho, S.H.; Liu, Z.; Hasunuma, T.; Chang, T.T.; Chang, K.F.; Chang, J.S.; Ren, N.Q.; Kondo, A. Improving carbohydrate production of Chlorella sorokiniana NIES-2168 through semi-continuous process coupled with mixotrophic cultivation. Biotechnol. J. 2016, 11, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Salla, A.C.V.; Margarites, A.C.; Seibel, F.I.; Holz, L.C.; Briao, V.B.; Bertolin, T.E.; Colla, L.M.; Costa, J.A.V. Increase in the carbohydrate content of the microalgae Spirulina in the culture by nutrient starvation and the addition of residues of whey protein concéntrate. Bioresour. Technol. 2016, 209, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, Q.; Guo, Q.; Zheng, W.; Wang, G.; Chen, L. Raceway pond cultivation of a new Arthrospira sp. ZJWST-S1 in digested piggery wastewater treated by MBR and ozonation. Int. J. Agric. Biol. Eng. 2017, 10, 115–124. [Google Scholar] [CrossRef]
- Daneshvar, E.; Antikainen, L.; Koutra, E.; Kornaros, M.; Bhatnagar, A. Investigation on the feasibility of Chlorella vulgaris cultivation in a mixture of pulp and aquaculture effluents: Treatment of wastewater and lipid extraction. Bioresour. Technol. 2018, 255, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.P.; Nguyen, D.H.; Lim, J.W.; Chang, C.K.; Leong, H.Y.; Tran, T.N.T.; Vu, T.B.H.; Nguyen, T.T.C.; Show, P.L. Investigation of the Relationship between Bacteria Growth and Lipid Production Cultivating of Microalgae Chlorella vulgaris in Seafood Wastewater. Energies 2019, 12, 2282. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, H.; Wang, Q. A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 2019, 275, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Chen, J.; Zhu, B.; Chen, X.; Basheer, S.; Cui, F.; Qian, J. Filamentous microalgae Tribonema sp. cultivation in the anaerobic/oxic effluents of petrochemical wastewater for evaluating the efficiency of recycling and treatment. Biochem. Eng. J. 2019, 145, 27–32. [Google Scholar] [CrossRef]
- Do, J.M.; Jo, S.W.; Kim, I.S.; Na, H.; Lee, J.H.; Kim, H.S.; Yoon, H.S. A Feasibility Study of Wastewater Treatment Using Domestic Microalgae and Analysis of Biomass for Potential Applications. Water 2019, 11, 2294. [Google Scholar] [CrossRef] [Green Version]
- Behl, K.; SeshaCharan, P.; Joshi, M.; Sharma, M.; Mathur, A.; Kareya, M.S.; Jutur, P.P.; Bhatnagar, A.; Nigam, S. Multifaceted applications of isolated microalgae Chlamydomonas sp. TRC-1 in wastewater remediation, lipid production and bioelectricity generation. Bioresour. Technol. 2020, 304, 122993. [Google Scholar] [CrossRef]
- Japar, A.S.; Takriff, M.S.; Yasin, N.H.M. Microalgae acclimatization in industrial wastewater and its effect on growth and primary metabolite composition. Algal Res. 2021, 53, 102163. [Google Scholar] [CrossRef]
- Farooq, W.; Suh, W.I.; Park, M.S.; Yang, J.W. Water use and its recycling in microalgae cultivation for biofuel application. Bioresour. Technol. 2015, 184, 73–81. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Eriksson, K.; Sellstedt, A. Mixotrophic and heterotrophic production of lipids and carbohydrates by a locally isolated microalga using wastewater as a growth medium. Bioresour. Technol. 2018, 257, 260–265. [Google Scholar] [CrossRef]
- Farroq, W.; Lee, Y.C.; Ryu, B.G.; Kim, B.H.; Kim, H.S.; Choi, Y.E.; Yang, J.W. Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity. Bioresour. Technol. 2013, 132, 230–238. [Google Scholar] [CrossRef]
- Dávila, B. Obtención de Bioetanol a Partir de Biomasa Microalgal Cultivada en Agua Residual Empleando Ozoflotación Como método de Cosecha. In Obtaining Bioethanol from Microalgal Biomass Cultivated in Wastewater Using Ozoflotation as the Harvesting Method; Tesis de Maestría en Ingeniería Ambiental; Universidad Nacional Autónoma de México: Distrito Federal, Mexico, 2016; Available online: https://repositorio.unam.mx/contenidos/3433052 (accessed on 17 May 2022).
- Tan, F.; Wang, Z.; Zhouyang, S.Y.; Li, H.; Xie, Y.P.; Wang, Y.P.; Zheng, Y.M.; Li, Q.B. Nitrogen and phosphorus removal coupled with carbohydrate production by five microalgae cultures cultivated in biogas slurry. Bioresour. Technol. 2016, 221, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Microalgal biomass generation by phycoremediation of dairy industry wastewater: An integrated approach towards sustainable biofuel production. Bioresour. Technol. 2016, 221, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Mata, S.N.; Santos, T.S.; Cardoso, L.G.; Andrade, B.B.; Duarte, J.H.; Costa, J.A.V.; de Souza, C.O.; Druzian, J.I. Spirulina sp. LEB 18 cultivation in a raceway-type bioreactor using wastewater from desalination process: Production of carbohydrate-rich biomass. Bioresour. Technol. 2020, 311, 123495. [Google Scholar] [CrossRef] [PubMed]
- Jambo, S.A.; Abdulla, R.; Azhar, S.H.M.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A review on third generation bioethanol feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Bhagea, R.; Bhoyroo, V.; Puchooa, D. Microalgae: The next best alternative to fossil fuels after biomass. A review. Microbiol. Res. 2019, 10, 12–24. [Google Scholar] [CrossRef] [Green Version]
- da Maia, J.L.; Cardoso, J.S.; Mastrantonio, D.J.S.; Bierhals, C.K.; Moreira, J.B.; Costa, J.A.V.; de Morais, M.G. Microalgae starch: A promising raw material for the bioethanol production. Int. J. Biol. Macromol. 2020, 165, 2739–2749. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, Y.D.; Chang, C.Y.; Lai, Y.Y.; Chen, C.Y.; Kondo, A.; Ren, N.Q.; Chang, J.S. Feasibility of CO2 mitigation and carbohydrate production by microalga Scenedesmus obliquus CNW-N used for bioethanol fermentation under outdoor conditions: Effects of seasonal changes. Biotechnol. Biofuels 2017, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Onay, M. Bioethanol production from Nannochloropsis gaditana in municipal wastewater. Energy Proc. 2018, 153, 253–257. [Google Scholar] [CrossRef]
- Chandra, N.; Shukla, P.; Mallick, N. Role of cultural variables in augmenting carbohydrate accumulation in the green microalga Scenedesmus acuminatus for bioethanol production. Biocatal. Agric. Biotechnol. 2020, 26, 101632. [Google Scholar] [CrossRef]
- Constantino, A.; Rodrigues, B.; Leon, R.; Barros, R.; Raposo, S. Alternative chemo-enzymatic hydrolysis strategy applied to different microalgae species for bioethanol production. Algal Res. 2021, 56, 102329. [Google Scholar] [CrossRef]
- Özcimen, D.; Kocer, A.T.; Inan, B.; Özer, T. Bioethanol production from microalgae. In Hanbook of Microalgae-Based Process and Products. Fundamentals and Advances in Energy, Food, Feed, Fertilizer, and Bioactive Compounds; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 373–389. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Synergy of biofuel production with waste remediation along with value-added co-products recovery through microalgae cultivation: A review of membrane-integrated green approach. Sci. Total Environ. 2020, 698, 134169. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell. Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. Available online: https://0-academic-oup-com.brum.beds.ac.uk/femsre/article/22/3/151/643945 (accessed on 20 June 2022). [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure–activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Radonic, A.; Thulke, S.; Achenbach, J.; Kurth, A.; Vreemann, A.; König, T.; Walter, C.; Possinger, K.; Nitsche, A. Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to vaccinia virus. J. Antivir. Antiretrovir. 2010, 2, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Kanekiyo, K.; Lee, J.B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an antiviral polysaccharide, Nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Colica, G.; De Philippis, R. Exopolysaccharides from cyanobacteria and their possible industrial applications. In Cyanobacteria: An Economic Perspective; Sharma, N.K., Rai, A.K., Stal, L.J., Eds.; Wiley Blackwell: West Sussex, UK, 2014; pp. 197–207. [Google Scholar] [CrossRef]
- Challouf, R.; Trabelsi, L.; Dhieb, R.B.; El Abed, O.; Yahia, A.; Ghozzi, K.; Ammar, J.B.; Omran, H.; Ouada, H.B. Evaluation of Cytotoxicity and Biological Activities in Extracellular Polysaccharides Released by Cyanobacterium Arthrospira platensis. Braz. Arch. Biol. Technol. 2011, 54, 831–838. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-inflammatory activity of exopolysaccharides from Phormidium sp. ETS05, the most abundant cyanobacterium of the therapeutic Euganean thermal muds, using the zebrafish model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campa-Córdova, A.I.; Hernández-Saavedra, N.Y.; De Philippis, R.; Ascencio, F. Generation of superoxide anion and SOD activity in haemocytes and muscle of American white shrimp (Litopenaeus vannamei) as a response to β-glucan and sulphated polysaccharide. Fish Shellfish Immunol. 2002, 12, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Gudmundsdottir, A.B.; Omarsdottir, S.; Brynjolfsdottir, A.; Paulsen, B.S.; Olafsdottir, E.S.; Freysdottir, J. Exopolysaccharides from Cyanobacterium aponinum from the blue lagoon in Iceland increase IL-10 secretion by human dendritic cells and their ability to reduce the IL 17+RORγt+/IL-10+ FoxP3+ ratio in CD4+ T cells. Immunol. Lett. 2015, 163, 157–162. [Google Scholar] [CrossRef]
- Liao, H.F.; Wu, T.J.; Tai, J.L.; Chi, M.C.; Lin, L.L. Immunomodulatory Potential of the Polysaccharide-Rich Extract from Edible Cyanobacterium Nostoc commune. Med. Sci. 2015, 3, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Yeh, H.Y.; Liao, Z.H.; Hung, S.W.; Chen, B.; Lee, P.T.; Nan, F.H.; Shih, W.L.; Chang, C.C.; Lee, M.C. An in vitro study shows the potential of Nostoc commune (Cyanobacteria) polysaccharides extract for wound-healing and anti-allergic use in the cosmetics industry. J. Funct. Foods 2021, 87, 104754. [Google Scholar] [CrossRef]
- Li, H.F.; Su, L.N.; Chen, S.; Zhao, L.B.; Wang, H.Y.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical characterization and functional analysis of the polysaccharide from the edible microalga Nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef] [Green Version]
- Ou, Y.; Xu, S.; Zhu, D.; Yang, X. Molecular mechanisms of exopolysaccharide from Aphanothece halophytica (EPSAH) induced apoptosis in HeLa cells. PLoS ONE 2014, 9, e87223. [Google Scholar] [CrossRef]
- Flores, C.; Lima, R.T.; Adessi, A.; Sousa, A.; Pereira, S.B.; Granja, P.L.; De Philippis, R.; Soares, P.; Tamagnini, P. Characterization and antitumor activity of the extracellular carbohydrate polymer from the cyanobacterium Synechosystis ΔsigF mutant. Int. J. Biol. Macromol. 2019, 136, 1219–1227. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Bioactivity and Applications of Polysaccharides from Marine Microalgae. In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2014; pp. 1–38. [Google Scholar] [CrossRef]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, P.M. Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Vambe, M.; Lovász, C.; Molnár, Z.; van Staden, J.; Ördög, V. Effect of cell disruption methods on the extraction of bioactive metabolites from microalgal biomass. J. Biotechnol. 2020, 307, 35–43. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Assar, D.H.; Abdelnaby, A.; Asa, S.A.; Abdelhiee, E.Y.; Ibrahim, S.S.; Abdel-Daim, M.M.; Almeer, R.; Atiba, A. Healing potential of Spirulina platensis for skin wounds by modulating bFGF, VEGF, TGF-ß1 and α-SMA genes expression targeting angiogenesis and scar tissue formation in the rat model. Biomed. Pharmacother. 2021, 137, 111349. [Google Scholar] [CrossRef] [PubMed]
- Komprda, T.; Sladek, Z.; Sevcikova, Z.; Svehlova, V.; Wijacki, J.; Guran, R.; Do, T.; Lackova, Z.; Polanska, H.; Vrlikova, L.; et al. Comparison of dietary oils with different polyunsaturated fatty acid n-3 and n-6 content in the rat model of cutaneous wound healing. Int. J. Mol. Sci. 2020, 21, 7911. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F.K.; Elgohary, R.; Salama, A. Amelioration of hepatic encephalopathy using Dunaliella salina microalgae in rats: Modulation of hyperammonemia/TLR4. BioMed Res. Int. 2021, 2021, 8843218. [Google Scholar] [CrossRef]
- Risjani, Y.; Mutmainnah, N.; Manurung, P.; Wulan, S.N. Yunianta Exopolysaccharide from Porphyridium cruentum (purpureum) is not toxic and stimulates immune response against vibriosis: The assessment using zebrafish and white shrimp Litopenaeus vannamei. Mar. Drugs 2021, 19, 133. [Google Scholar] [CrossRef]
- Carballo, C.; Mateus, A.P.; Maya, C.; Mantecón, L.; Power, D.M.; Manchado, M. Microalgal extracts induce larval programming and modify growth and the immune response to bioactive treatments and LCDV in Senegalese sole post-larvae. Fish Shellfish Immunol. 2020, 106, 263–272. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira-Garcia, F.A.; Sales-Campos, H.; Yuen, V.G.; Machado, J.R.; de Barros-Viana, G.S.; Freire-Oliveira, C.J.; McNeill, J.H. Arthrospira (Spirulina) platensis attenuates dextran sulfate sodium-induced colitis in mice by suppressing key pro-inflammatory cytokines. Korean J. Gastroenterol. 2020, 76, 150–158. [Google Scholar] [CrossRef]
- Yang, Y.P.; Tong, Q.Y.; Zheng, S.H.; Zhou, M.D.; Zeng, Y.M.; Zhou, T.T. Anti-inflammatory effect of fucoxanthin on dextran sulfate sodium-induced colitis in mice. Nat. Prod. Res. 2020, 34, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Li, W.L.; Hua, S.Y.; Qi, Y.C.; Xie, T.T.; Qiao, Y.; Zhou, M. Calcium phosphate engineered photosynthetic microalgae to combat hypoxic-tumor by in-situ modulating hypoxia and cascade radio-phototherapy. Theranostics 2021, 11, 3580–3594. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Lin, Y.J.; Liu, W.; Lin, H.Y.; Chou, H.Y.; Thia, C.; Wu, J.H.; Chang, J.S.; Wen, Z.H.; Chang, J.J.; et al. Metabolic engineering probiotic yeast produces 3S, 3′S-astaxanthin to inhibit B16F10 metastasis. Food Chem. Toxicol. 2020, 135, 110993. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.S.; Miller, D.D.; Padilla-Zakour, O.I.; Lei, X.G. Supplemental microalgal iron helps replete blood hemoglobin in moderately anemic mice fed a rice-based diet. Nutrients 2020, 12, 2239. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.B.; Katanić-Stanković, J.S.; Mihailović, V.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Algae as a source of bioactive compounds to prevent the development of type 2 Diabetes Mellitus. Curr. Med. Chem. 2021, 28, 4592–4615. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al-Kaabi, J. Epidemiology of type 2 Diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Setyaningsih, I.; Prasetyo, H.; Agungpriyono, D.R.; Tarman, K. Antihyperglycemic activity of Porphyridium cruentum biomass and extra-cellular polysaccharide in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2020, 156, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pliego, L.E.; Martínez-Carrillo, B.E.; Reséndiz-Albor, A.A.; Valdés-Ramos, R. Effect on adipose tissue of diabetic mice supplemented with n-3 fatty acids extracted from microalgae. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 728–735. [Google Scholar] [CrossRef]
- Zainul-Azlan, N.; Mohd-Yusof, Y.A.; Makpol, S. Chlorella vulgaris ameliorates oxidative stress and improves the muscle regenerative capacity of young and old Sprague-Dawley rats. Nutrients 2020, 12, 3752. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, T.C.; Cazarin, C.B.B.; Maróstica, M.R.J.; Zerlotti-Mercadante, A.; Jacob-Lopes, E.; Zepka, L.Q. Microalgae carotenoids intake: Influence on cholesterol levels, lipid peroxidation and antioxidant enzymes. Food Res. Int. 2020, 128, 108770. [Google Scholar] [CrossRef]
- Stirk, W.A.; van Staden, J. Bioprospecting for bioactive compounds in microalgae: Antimicrobial compounds. Biotechnol. Adv. 2022, 59, 107977. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of Wastewater by Microalgae and the Potential Applications of the Produced Biomass—A Review. Water 2021, 13, 27. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; Rodríguez-Miranda, E.; Gómez-Serrano, C.; González-López, C.V. Year-long evaluation of microalgae production in wastewater using pilot-scale raceway photobioreactors: Assessment of biomass productivity and nutrient recovery capacity. Algal Res. 2021, 60, 102500. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bagnani, M.; Soon, W.L.; Mezzenga, R. Turning Food Protein Waste into Sustainable Technologies. Chem. Rev. 2022, 35772093. [Google Scholar] [CrossRef]
- Kharytonov, M.M.; Martynova, N.V.; Babenko, M.G.; Rula, I.V. Environmentally compatible utilization of reclaimed minelands for sustainable production food and bioenergy feedstock. In Actual Problemas of Natural Sciences: Modern Scientific Discussions. Collective Monograph; Bairamova, O.V., Baranovych, D.B., et al., Eds.; Baltija Publishing: Riga, Latvia, 2020; pp. 625–658. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Mykolenko, S.; Liedienov, V.; Kharytonov, M.; Makieieva, N.; Kuliush, T.; Queralt, I.; Marguí, E.; Hidalgo, M.; Pardini, G.; Gispert, M. Presence, mobility and bioavailability of toxic metal(oids) in soil, vegetation and water around a Pb-Sb recycling factory (Barcelona, Spain). Environ. Pollut. 2018, 237, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, Y.; Wang, C.; Guo, J.; Zhou, C.; Zhang, R.; Ma, Y.; Ma, X.; Wang, L.; Cheng, Y.; et al. Integrated marine microalgae biorefineries for improved bioactive compounds: A review. Sci. Total Environ. 2022, 817, 152895. [Google Scholar] [CrossRef] [PubMed]
- Mastropetros, S.G.; Pispas, K.; Zagklis, D.; Ali, S.S.; Kornaros, M. Biopolymers production from microalgae and cyanobacteria cultivated in wastewater: Recent advances. Biotechnol. Adv. 2022, 60, 107999. [Google Scholar] [CrossRef]
- Arias, D.M.; García, J.; Uggetti, E. Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. New Biotechnol. 2020, 55, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Ng, E.A.S.; Chen, W.H.; Culaba, A.B.; Kwon, E.E. Life cycle assessment of microalgal biorefinery: A state-of-the-art review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
- Arsova, S.; Genovese, A.; Ketikidis, P.H. Implementing circular economy in a regional context: A systematic literature review and a research agenda. J. Clean. Prod. 2022, 133117. [Google Scholar] [CrossRef]



| Pigment | Microalgae/Cyanobacteria Species | Country | Bioprocess Operational Strategy | Pigment Content/Productivity | Reference |
|---|---|---|---|---|---|
| Phycocyanin | Nostoc commune TUBT05 Oscillatoria okeni TISTR8549 | Thailand | Batch | 543.7 ± 28.78 mg g−1 | [38] |
| Leptolyngbya sp. QUCCCM56 | Qatar | Batch | 169.9 ± 3.6 mg g−1 | [39] | |
| Arthrospira (Spirulina) maxima LJGR 1 | Mexico | Pilot-plant level | 95–135 mg g−1 | [40] | |
| Astaxanthin | Haematococcus pluvialis | China | Batch | 140 mg m−2 d−1 | [41] |
| Chromochloris zofingiensis | China | Batch | 38.9 mg L−1 | [42] | |
| Chlorella sorokiniana IBRC-M50023 | Iran | Batch | 24.2 ± 0.1 mg L−1 | [43] | |
| Haematococcus pluvialis NIES-144 | Republic of Korea | Batch | 10.3 ± 0.4 mg L−1 | [44] | |
| Lutein | Chlorella sorokiniana SAG 211/8K | The Netherlands | Batch | 7.3 ± 0.5 mg g−1 | [45] |
| Chlorella sorokiniana MB-I-M12 | Taiwan | Semi-continuous | 4.46 mg L−1 d−1 | [46] | |
| Chlorella sp. GY-H4 | China | Semi-continuous | 38.5–63 mg L−1 | [47] | |
| Koliella antárctica Tetraselmis chui | Italy | Batch | 0.27–2.05mg g−1 0.29–2.2 mg g−1 | [48] | |
| Chlorella sp. AE10 | China | Batch | 4.4 mg L d−1 (red light) 9.58 mg g−1 (blue light) | [49] | |
| Allophycocyanin | Arthrospira platensis | Brazil | Fed batch | 13.3 mg g−1 | [50] |
| Phycoerytrin | 4.1 mg g−1 | ||||
| Violaxanthin | Nannochloropsis gatidana | Italy | Batch | 0.24–2.07 mg g−1 | [48] |
| Chlorophyll | Chlorella sorokiniana SAG 211/8K | The Netherlands | Batch | 35.4 1.7 mg g−1 | [45] |
| Nannochloropsis gatidana | Italy | Batch | 1.8–13.8 mg g−1 | [48] |
| Pigment | Microalgae Specie | Wastewater Type | Bioprocess Operational Strategy | Pigment Content/Productivity | Reference |
|---|---|---|---|---|---|
| Phycocyanin | Arthrospira platensis | Palm oil municipal effluent | Fed batch | 120.13 ± 1.1 mg g−1 | [56] |
| Nostoc sp. Arthrospira platensis Porphyridium purpureum | Industrial | Batch | 103 mg g−1 | [64] | |
| Astaxanthin | Haematococcus pluvialis | Potato juice | Batch | 14.7–27.9 mg g−1 | [62] |
| Lutein | Chlorella sorokiniana | Industrial and municipal | Batch/Continuous | 1.03 mg g−1 | [59] |
| Chlorella sp. GY-H4 | Diluted food waste hydrolysate | Semi-continuous | 44 mg L−1 (with an initial glucose concentration of 10 g L−1) 63 mg L−1 (with an initial glucose concentration of 20 g L−1) | [47] | |
| β-carotene | Desmodesmus spp. | Industrial | Batch | * 0.6473 mg g−1 | [60] |
| Dunaliella salina | * 0.7435 mg g−1 | ||||
| Nannochloropsis limnetica | * 0.7435 mg g−1 | ||||
| Chlorella sorokiniana | * 1.039 mg g−1 | ||||
| Nephroselmis sp. | Industrial | Batch | * 0.188 mg g−1 | [61] | |
| Dunaliella sp. FACHB-558 | Poultry litter | Batch | 7.26 mg L−1 | [63] | |
| Violaxanthin | Desmodesmus spp. | Industrial | Batch | * 0.0546 mg g−1 | [60] |
| Dunaliella salina | * 0.08301 mg g−1 | ||||
| Nannochloropsis limnetica | * 1.228 mg g−1 | ||||
| Nannochloropsis salina | * 1.679 mg g−1 | ||||
| Allophycocyanin | Nostoc sp. Arthrospira platensis Porphyridium purpureum | Industrial | Batch | 57 mg g−1 | [64] |
| Phycoerytrin | 30 mg g−1 | ||||
| Chlorophyll (Chl) | Botryococcus braunii | Municipal | Batch | (Chl-a) 32.21 µg ml−1 (Chl-b) 50.51 µg ml−1 | [57] |
| Chlorella sorokiniana | Industrial and municipal | Batch/Continuous | 18.01 mg L−1 d−1 | [59] |
Advantages
|
| Microalgae Species | Culture Conditions | Macromolecule (% dry w/w) | Reference | ||
|---|---|---|---|---|---|
| Carbohydrate | Protein | Lipid | |||
| N. oleabundans | Nutrient deficiency induced using various percentages of anaerobic digestates of vinasse and the addition of NaHCO3 (under controlled conditions) and three different culture media in flat plate photobioreactors (under uncontrolled conditions) resulted in lipid accumulation | - | - | 18–39 | [26] |
| Spirulina platensis LEB 52 | Nutrient deficiency induced by using a Zarrouk medium diluted to 20% amended with 2.5% of two different residues from the ultra and nanofiltration of whey protein. | 45–58 | 51 | - | [112] |
| Arthrospira sp. ZJWST | Raceways pond cultivation under nutrient limitation induced by the use of digested piggery wastewater pre-treated with a membrane bioreactor and ozonation amended with NaHCO3. | - | 59 | - | [113] |
| Chlorococcum sp. | Batch culture under nitrogen starvation using a digestate from pig manure as a nutrient source under indoor and outdoor conditions. | 42–45 | 16–20 | 11–13 | [52] |
| Scenedesmus sp. | Nutrient limitation induced by different dilution rates, centrate (from the anaerobic digestion of urban wastewater) percentages, and culture depths. | - | - | 10 | [21] |
| Chlorella vulgaris | Nutrient deficiency induced by different dilution percentages by mixing natural lake water, aquaculture wastewater (AW) and pulp wastewater (PW). The experimental units with the highest growth were selected for microalgae culture in AW and PW without nutrient addition. | 18–19 | 44–46 | 44–50 | [114] |
| Chlorella vulgaris | Different initial microalgae density (20–65 mg L−1) and nitrogen starvation during 14 days of microalgae culture. | - | - | 32 | [115] |
| Chlorella vulgaris | Co-cultivation of microalgae with filamentous fungi was used for molasses wastewater treatment. This strategy was carried out by inoculating microalgae and fungi in wastewater under the optimized conditions (temperature and inoculation ratio on biomass production). | - | 62 | 22 | [116] |
| Tribonema sp. | The algae cultivation was performed using open vertical tubular PBR with an initial culture density of 0.2 g L−1. Nutrient starvation was induced by the differences in the chemical composition of petrochemical wastewater effluents (used directly without further treatment). | 55 | >20 | 34–36 | [117] |
| T. obliquus KNUA019 A. quadricellulare KNUA020 Desmodesmus sp. KNUA024 Pseudopediastrum sp. KNUA039 | Microalgae strains pellets were suspended with filtered municipal wastewater and inoculated 10% into 1 L sterile flasks containing filtered treated primary settled wastewater for 11 days. | 24–28 | 32–40 | 16–26 | [118] |
| Chlamydomonas sp. TRC-1 | Nutrient starvation was induced by the differences in the chemical composition of effluents (GB-11 medium and textile effluents). | 19 | 52 | 11 | [119] |
| Chlorella vulgaris Chlorella sorokiniana UKM3 Chlamydomonas sp. UKM6 Scenedesmus sp. UKM9 | Nitrogen depletion through acclimatization and continuous light exposure in anaerobic digested palm oil mill effluent (POME). Microalgae were cultured in anaerobic digested POME without any dilution or additional substrate. | 21–50 | [120] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology 2022, 11, 1146. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11081146
Olguín EJ, Sánchez-Galván G, Arias-Olguín II, Melo FJ, González-Portela RE, Cruz L, De Philippis R, Adessi A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology. 2022; 11(8):1146. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11081146
Chicago/Turabian StyleOlguín, Eugenia J., Gloria Sánchez-Galván, Imilla I. Arias-Olguín, Francisco J. Melo, Ricardo E. González-Portela, Lourdes Cruz, Roberto De Philippis, and Alessandra Adessi. 2022. "Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds" Biology 11, no. 8: 1146. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11081146