Importance of Magnesium Status in COVID-19
Abstract
:Simple Summary
Abstract
1. Introduction
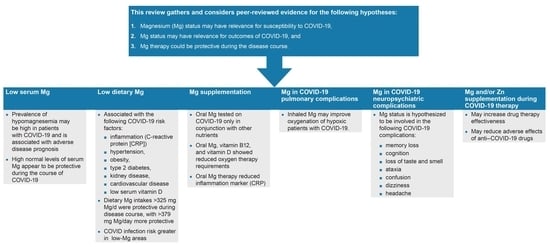
2. Low Serum Mg and COVID-19
Low Serum Mg and Risk of Developing Severe COVID-19 in Hospitalized Patients
3. Low Dietary Mg Intake and COVID-19
4. Mg Supplements and COVID-19
5. Mechanisms of Action of Mg in Pulmonary Complications of COVID-19
6. Mg in Neurological and Psychiatric Complication of COVID-19
6.1. Guillain-Barré Syndrome and Encephalopathies
6.2. Memory and Cognition
- increased neuroplasticity [151],
- upregulation of cAMP response element-binding protein (CREB)-mediated transcription,
- modulation of the activity of some transcription factors (c-Fos, nuclear factor-κB [NF-κB]),
- increased brain-derived neurotrophic factor (BDNF) action,
- reduced oxidative stress in the brain,
- increased reduced glutathione levels, or
- reduced proinflammatory cytokine synthesis and release [23].
6.3. Taste and Gustatory Dysfunction, Loss of Smell, and Loss of Appetite
6.4. Ataxia
6.5. Confusion, Delirum, and Consciousness Disturbances
6.6. Cranial Nerve Deficits and Cranial Nerve Palsy
6.7. Convulsions, Child Epilepsy, and Hallucinations
6.8. Demyelination and Axonal Neuropathies
6.9. Headache and Dizziness
- by increasing the amount of circulating proinflammatory cytokines (Il-1β, TNF-α, and others) that enhance trigeminal nociception [201];
- through direct action of the virus on trigeminal nerve endings;
- by local hypoxic phenomena that also affect the peripheral trigeminal endings [130]; and
- by increasing oxidative stress and free radical formation.
- by reducing cerebral and pericranial vascular smooth muscle spasms, modulating the NO level in the cell, and eliminating NO trapped inside the cell [202]; and
- by inhibiting IL-1β and TNF synthesis and reducing neuroinflammation.
6.10. Immunity
7. Interrelationships between Mg, Zn, and Agents Used to Treat COVID-19
- pharmacodynamic interactions between Mg2+ and Zn2+ and the action of anti-COVID-19 drugs, including a) direct influence on the drug mechanism of action and b) indirect influence through the influence on the body immunity of patients with COVID-19;
- pharmacokinetic interactions (related to the influence of Mg and Zn on the absorption, transport in the blood, and elimination from the body) of the drugs used;
- influence of anti-COVID-19 medication on the plasma or tissue concentration of these two cations; and
- influence of Mg2+ and Zn2+ on the adverse effects of anti-COVID-19 medication.
- 5.
- Among the numerous drugs that are or have been used to treat COVID-19, these interrelations are partially known only for some. Future studies are needed.
- 6.
- Many drugs that have been or are used in anti-COVID-19 therapy are also used to treat other diseases. Their pharmacokinetic characteristics and mechanism of action remain the same and are intrinsically determined by their molecular structure. Some pharmacokinetic characteristics could be modified if new pharmaceutical forms are used.
- 7.
- Frequently, patients with COVID-19 receive not only anti-COVID-19 therapy but also treatment for preexisting chronic diseases. Drugs used to treat these conditions can change Mg and Zn plasma concentrations and thus indirectly influence anti-COVID-19 therapy.
- 8.
- Extracellular and intracellular Mg levels and Zn plasma levels in hospitalized patients with COVID-19 are highly variable. Many patients have hypomagnesemia or hypozincemia (or both) upon admission, and many develop imbalances of these elements during the disease course.
- 9.
- Determinations of serum Mg and Zn concentrations at admission and during hospitalization are inconsistent (and in many cases, only sporadically undertaken).
- 10.
- Oral nutrition of these patients can be deficient, and the solutions administered parenterally rarely aim to correct Mg deficiency.
7.1. Pharmacodynamic Interactions
7.2. Pharmacokinetic Interactions
7.3. Influence of Anti-COVID-19 Medication on Plasma or Tissue Mg2+ and Zn2+ Concentrations
7.4. Influence of Mg and Zn on Adverse Effects of Anti-COVID-19 Medication
8. Discussion, Conclusions, and Future Directions
- COVID-19 infection risk is shown to be higher in areas of low environmental Mg [105].
- Pulmonary inhalation of Mg improves oxygenation in patients with COVID-19 [126].
- Low Mg status and hypomagnesemia are associated with the neuropsychiatric complications seen with COVID-19 (see Section 6 in this review).
- SARS-CoV-2 infection might be a cause of hypomagnesemia (this review).
- Mg as well as Zn may be beneficial in COVID-19 therapy by enhancing the effects of medications or diminishing their side effects (see Section 7 in this review).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int (accessed on 3 April 2023).
- Sridhar, S.; Nicholls, J. Pathophysiology of infection with SARS-CoV-2: What is known and what remains a mystery. Respirology 2021, 26, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-specific immune response and the pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef] [PubMed]
- García-Escobar, A.; Vera-Vera, S.; Jurado-Román, A.; Jiménez-Valero, S.; Galeote, G.; Moreno, R. Calcium signaling pathway is involved in the shedding of ACE2 catalytic ectodomain: New insights for clinical and therapeutic applications of ACE2 for COVID-19. Biomolecules 2022, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Cavezzi, A.; Menicagli, R.; Troiani, E.; Corrao, S. COVID-19, cation dysmetabolism, sialic acid, CD147, ACE2, viroporins, hepcidin and ferroptosis: A possible unifying hypothesis. F1000Research 2022, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, T.; Alghanem, B.; Shaibah, H.; Mansour, F.A.; Alamri, H.S.; Akiel, M.A.; Alroqi, F.; Boudjelal, M. SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1-like-macrophages: Potential role of angiotensin-converting enzyme inhibitor (perindopril). Front. Immunol. 2021, 12, 728896. [Google Scholar] [CrossRef]
- Taheri, M.; Bahrami, A.; Habibi, P.; Nouri, F. A review on the serum electrolytes and trace elements role in the pathophysiology of COVID-19. Biol. Trace Elem. Res. 2021, 199, 2475–2481. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Razzaque, M.S. COVID-19 pandemic: Can maintaining optimal zinc balance enhance host resistance? Tohoku J. Exp. Med. 2020, 251, 175–181. [Google Scholar] [CrossRef]
- Wilck, N.; Balogh, A.; Markó, L.; Bartolomaeus, H.; Müller, D.N. The role of sodium in modulating immune cell function. Nat. Rev. Nephrol. 2019, 15, 546–558. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, H.; Hu, J.; Lian, J.; Gu, J.; Zhang, S.; Ye, C.; Lu, Y.; Jin, C.; Yu, G.; et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020, 94, 81–87. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Alfano, G.; Ferrari, A.; Fontana, F.; Perrone, R.; Mori, G.; Ascione, E.; Magistroni, R.; Venturi, G.; Pederzoli, S.; Margiotta, G.; et al. Hypokalemia in Patients with COVID-19. Clin. Exp. Nephrol. 2021, 25, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Feske, S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007, 7, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Raesi, A.; Saedi Dezaki, E.; Moosapour, H.; Saeidifard, F.; Habibi, Z.; Rahmani, F.; Kheiri, S.; Taheri, E. Hypocalcemia in COVID-19: A prognostic marker for severe disease. Iran. J. Pathol. 2021, 16, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Martha, J.W.; Wibowo, A.; Pranata, R. Hypocalcemia is associated with severe COVID-19: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 337–342. [Google Scholar] [CrossRef]
- Hadi, J.M.; Hassan, S.M.J.; Saeed, M.M.M.; Hussein, B.K.; Ali, B.M.; Muhamad, L.E.; Abdullah, A.J.; Ali, N.N.; Rahman, H.A.; Sofihussein, H.Q.; et al. Estimation of serum calcium on the severity and mortality in COVID-19 infections in Sulaymaniyah City, Kurdistan Region of Iraq: A cross-sectional study. Clin. Pract. 2022, 12, 1001–1008. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Alemzadeh, E.; Ziaee, M.; Abedi, A.; Salehiniya, H. The effect of low serum calcium level on the severity and mortality of COVID patients: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2021, 9, 1219–1228. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Sarandi, E. Micronutrient deficiencies in patients with COVID-19: How metabolomics can contribute to their prevention and replenishment. BMJ Nutr. Prev. Health 2020, 3, 419–420. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Sarandi, E.; Georgaki, S. The snapshot of metabolic health in evaluating micronutrient status, the risk of infection and clinical outcome of COVID-19. Clin. Nutr. ESPEN 2021, 44, 173–187. [Google Scholar] [CrossRef]
- Gout, E.; Rébeillé, F.; Douce, R.; Bligny, R. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg2+ in cell respiration. Proc. Natl. Acad. Sci. USA 2014, 111, E4560–E4567. [Google Scholar] [CrossRef]
- Wolf, F.I.; Maier, J.A.; Rosanoff, A.; Barbagallo, M.; Baniasadi, S.; Castiglioni, S.; Cheng, F.C.; Day, S.C.; Costello, R.B.; Dominguez, L.J.; et al. The Magnesium Global Network (MaGNet) to promote research on magnesium in diseases focusing on COVID-19. Magnes. Res. 2021, 34, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Iotti, S.; Wolf, F.; Mazur, A.; Maier, J.A. The COVID-19 pandemic: Is there a role for magnesium? Hypotheses and perspectives. Magnes. Res. 2020, 33, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Combating COVID-19 and building immune resilience: A potential role for magnesium nutrition? J. Am. Coll. Nutr. 2020, 39, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Flink, E.B. Magnesium deficiency. Etiology and clinical spectrum. Acta Med. Scand. Suppl. 1981, 647, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Rosanoff, A.; Baniasadi, S.; Barbagallo, M.; Castiglioni, S.; Guerrero-Romero, F.; Iotti, S.; Mazur, A.; Micke, O.; Pourdowlat, G.; et al. The relevance of magnesium homeostasis in COVID-19. Eur. J. Nutr. 2022, 61, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Gerosa, C.; Nurchi, V.M.; Manchia, M.; Saba, L.; Coghe, F.; Crisponi, G.; Gibo, Y.; Van Eyken, P.; Fanos, V.; et al. The role of magnesium in pregnancy and in fetal programming of adult diseases. Biol. Trace Elem. Res. 2021, 199, 3647–3657. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Intestinal absorption and factors influencing bioavailability of magnesium—An update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef]
- Konrad, M.; Schlingmann, K.P.; Gudermann, T. Insights into the molecular nature of magnesium homeostasis. Am. J. Physiol. Ren. Physiol. 2004, 286, F599–F605. [Google Scholar] [CrossRef]
- Rubin, H. Magnesium: The missing element in molecular views of cell proliferation control. Bioessays 2005, 27, 311–320. [Google Scholar] [CrossRef]
- Kubota, T.; Shindo, Y.; Tokuno, K.; Komatsu, H.; Ogawa, H.; Kudo, S.; Kitamura, Y.; Suzuki, K.; Oka, K. Mitochondria are intracellular magnesium stores: Investigation by simultaneous fluorescent imagings in PC12 cells. Biochim. Biophys. Acta 2005, 1744, 19–28. [Google Scholar] [CrossRef]
- Iotti, S.; Frassineti, C.; Sabatini, A.; Vacca, A.; Barbiroli, B. Quantitative mathematical expressions for accurate in vivo assessment of cytosolic [ADP] and DeltaG of ATP hydrolysis in the human brain and skeletal muscle. Biochim. Biophys. Acta 2005, 1708, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Sargenti, A.; Castiglioni, S.; Olivi, E.; Bianchi, F.; Cazzaniga, A.; Farruggia, G.; Cappadone, C.; Merolle, L.; Malucelli, E.; Ventura, C.; et al. Magnesium deprivation potentiates human mesenchymal stem cell transcriptional remodeling. Int. J. Mol. Sci. 2018, 19, 1410. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.M.; Grande, A.; Frassineti, C. Magnesium is a key regulator of the balance between osteoclast and osteoblast differentiation in the presence of vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in infectious diseases in older people. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef]
- Brandao, K.; Deason-Towne, F.; Perraud, A.L.; Schmitz, C. The role of Mg2+ in immune cells. Immunol. Res. 2013, 55, 261–269. [Google Scholar] [CrossRef]
- de Jesus, J.R.; Galazzi, R.M.; Lopes Júnior, C.A.; Arruda, M.A.Z. Trace element homeostasis in the neurological system after SARS-CoV-2 infection: Insight into potential biochemical mechanisms. J. Trace Elem. Med. Biol. 2022, 71, 126964. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Chaigne-Delalande, B.; Li, F.Y.; O’Connor, G.M.; Lukacs, M.J.; Jiang, P.; Zheng, L.; Shatzer, A.; Biancalana, M.; Pittaluga, S.; Matthews, H.F.; et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 2013, 341, 186–191. [Google Scholar] [CrossRef]
- Weglicki, W.B. Hypomagnesemia and inflammation: Clinical and basic aspects. Annu. Rev. Nutr. 2012, 32, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Lötscher, J.; Martí, I.L.A.A.; Kirchhammer, N.; Cribioli, E.; Giordano Attianese, G.M.P.; Trefny, M.P.; Lenz, M.; Rothschild, S.I.; Strati, P.; Künzli, M.; et al. Magnesium sensing via LFA-1 regulates CD8+ T cell effector function. Cell 2022, 185, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Aslani, S.; Ataollahi, M.; Mahmoudi, M. The role of magnesium in different inflammatory diseases. Inflammopharmacology 2019, 27, 649–661. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A. Low magnesium and atherosclerosis: An evidence-based link. Mol. Aspects Med. 2003, 24, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Seelig, M.S.A.R. The Magnesium Factor; Avery Penguin Group: New York, NY, USA, 2003. [Google Scholar]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the diagnosis of magnesium status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Micke, O.; Vormann, J.; Kraus, A.; Kisters, K. Serum magnesium: Time for a standardized and evidence-based reference range. Magnes. Res. 2021, 34, 84–89. [Google Scholar] [CrossRef]
- Quilliot, D.; Bonsack, O.; Jaussaud, R.; Mazur, A. Dysmagnesemia in COVID-19 cohort patients: Prevalence and associated factors. Magnes. Res. 2020, 33, 114–122. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Mercado, M.; Rodriguez-Moran, M.; Ramírez-Renteria, C.; Martínez-Aguilar, G.; Marrero-Rodríguez, D.; Ferreira-Hermosillo, A.; Simental-Mendía, L.E.; Remba-Shapiro, I.; Gamboa-Gómez, C.I.; et al. Magnesium-to-calcium ratio and mortality from COVID-19. Nutrients 2022, 14, 1686. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wei, Z.; Zhang, H.; Wang, J.; Jia, R.; Zhou, M.; Li, X.; Zhang, H.; Chen, X.; Yu, Z.; et al. An interpretable machine learning model based on a quick pre-screening system enables accurate deterioration risk prediction for COVID-19. Sci. Rep. 2021, 11, 23127. [Google Scholar] [CrossRef] [PubMed]
- Beigmohammadi, M.T.; Bitarafan, S.; Abdollahi, A.; Amoozadeh, L.; Salahshour, F.; Mahmoodi Ali Abadi, M.; Soltani, D.; Motallebnejad, Z.A. The association between serum levels of micronutrients and the severity of disease in patients with COVID-19. Nutrition 2021, 91–92, 111400. [Google Scholar] [CrossRef]
- Li, P.; Lee, Y.; Jehangir, Q.; Lin, C.H.; Krishnamoorthy, G.; Sule, A.A.; Halabi, A.R.; Patel, K.; Poisson, L.; Nair, G.B. SARS-CoV-ATE risk assessment model for arterial thromboembolism in COVID-19. Sci. Rep. 2022, 12, 16176. [Google Scholar] [CrossRef]
- Rubeiz, G.J.; Thill-Baharozian, M.; Hardie, D.; Carlson, R.W. Association of hypomagnesemia and mortality in acutely ill medical patients. Crit. Care Med. 1993, 21, 203–209. [Google Scholar] [CrossRef]
- Velissaris, D.; Karamouzos, V.; Pierrakos, C.; Aretha, D.; Karanikolas, M. Hypomagnesemia in critically ill sepsis patients. J. Clin. Med. Res. 2015, 7, 911–918. [Google Scholar] [CrossRef]
- Alamdari, N.M.; Afaghi, S.; Rahimi, F.S.; Tarki, F.E.; Tavana, S.; Zali, A.; Fathi, M.; Besharat, S.; Bagheri, L.; Pourmotahari, F.; et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J. Exp. Med. 2020, 252, 73–84. [Google Scholar] [CrossRef]
- Lo Piano, F.; Corsonello, A.; Corica, F. Magnesium and elderly patient: The explored paths and the ones to be explored: A review. Magnes. Res. 2019, 32, 1–15. [Google Scholar] [CrossRef]
- Gommers, L.M.; Hoenderop, J.G.; Bindels, R.J.; de Baaij, J.H. Hypomagnesemia in type 2 diabetes: A vicious circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Flores-García, A.; Saldaña-Guerrero, S.; Simental-Mendía, L.E.; Rodríguez-Morán, M. Obesity and hypomagnesemia. Eur. J. Intern. Med. 2016, 34, 29–33. [Google Scholar] [CrossRef]
- Tin, A.; Grams, M.E.; Maruthur, N.M.; Astor, B.C.; Couper, D.; Mosley, T.H.; Selvin, E.; Coresh, J.; Kao, W.H. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015, 87, 820–827. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S. Association of hypomagnesemia with cardiovascular diseases and hypertension. Int. J. Cardiol. Hypertens. 2019, 1, 100005. [Google Scholar] [CrossRef]
- Ali, A.A.; Bakr, R.M.; Yousif, M.; Foad, R.E. Assessment of serum magnesium level in patients with bronchial asthma. Egypt. J. Chest Dis. Tuberc. 2015, 64, 535–539. [Google Scholar] [CrossRef]
- Li, Y.; Ashcroft, T.; Chung, A.; Dighero, I.; Dozier, M.; Horne, M.; McSwiggan, E.; Shamsuddin, A.; Nair, H. Risk factors for poor outcomes in hospitalised COVID-19 patients: A systematic review and meta-analysis. J. Glob. Health 2021, 11, 10001. [Google Scholar] [CrossRef] [PubMed]
- Damayanthi, H.; Prabani, K.I.P. Nutritional determinants and COVID-19 outcomes of older patients with COVID-19: A systematic review. Arch. Gerontol. Geriatr. 2021, 95, 104411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Bao, X.; Bi, J.; Lin, Y.; Shan, C.; Fan, X.; Bian, J.; Wang, X. Serum magnesium in patients with severe acute respiratory syndrome coronavirus 2 from Wuhan, China. Magnes. Res. 2021, 34, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Vijayaraghavan, K.; Khera, S.; Sica, D.A.; Frishman, W.H. Role of magnesium in cardiovascular diseases. Cardiol. Rev. 2014, 22, 182–192. [Google Scholar] [CrossRef]
- Gunay, S.; Caliskan, S.; Sigirli, D. Relationship of magnesemia with myocardial damage and mortality in patients with COVID-19. Magnes. Res. 2021, 34, 93–102. [Google Scholar] [CrossRef]
- Gourgoulianis, K.I.; Chatziparasidis, G.; Chatziefthimiou, A.; Molyvdas, P.A. Magnesium as a relaxing factor of airway smooth muscles. J. Aerosol Med. 2001, 14, 301–307. [Google Scholar] [CrossRef]
- Díez, J.J.; Iglesias, P.; García, A.; Martín-Casasempere, I.; Bernabéu-Andréu, F.A. Serum calcium, magnesium, and phosphorus levels in patients with COVID-19: Relationships with poor outcome and mortality. Horm. Metab. Res. 2023, 55, 31–39. [Google Scholar] [CrossRef]
- Ko, J.Y.; Danielson, M.L.; Town, M.; Derado, G.; Greenlund, K.J.; Kirley, P.D.; Alden, N.B.; Yousey-Hindes, K.; Anderson, E.J.; Ryan, P.A.; et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin. Infect. Dis. 2021, 72, e695–e703. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Heidari, A.; Johnson, R.H.; Advani, S.; Petersen, G. Serum magnesium levels in hospitalized patients with SARS-CoV-2. J. Investig. Med. 2022, 70, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Sarvazad, H.; Cahngaripour, S.H.; Eskandari Roozbahani, N.; Izadi, B. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. New Microbes New Infect. 2020, 38, 100807. [Google Scholar] [CrossRef] [PubMed]
- Nuoranne, P. On the reliability of the magnesium serum value as an indicator of body magnesium status. Nord. Vet. Med. 1978, 30, 71–73. [Google Scholar]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in aging, health and diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhou, Z.; Lin, T.; Wang, X. Magnesium depletion score is associated with long-term mortality in chronic kidney diseases: A prospective population-based cohort study. J. Nephrol. 2023, 36, 755–765. [Google Scholar] [CrossRef]
- Srivastava, S.; Rathor, R.; Singh, S.; Kumar, B.; Suryakumar, G. Obesity: A risk factor for COVID-19. Adv. Exp. Med. Biol. 2021, 1352, 195–210. [Google Scholar] [CrossRef]
- Nielsen, F.H. The problematic use of Dietary Reference Intakes to assess magnesium status and clinical importance. Biol. Trace Elem. Res. 2019, 188, 52–59. [Google Scholar] [CrossRef]
- Amin, M.N.; Bahoosh, S.R.; Eftekhari, M.; Hosseinzadeh, L. Herbal sources of magnesium as a promising multifaceted intervention for the management of COVID-19. Nat. Prod. Commun. 2022, 17, 1934578X221116235. [Google Scholar] [CrossRef]
- Arshad, M.S.; Khan, U.; Sadiq, A.; Khalid, W.; Hussain, M.; Yasmeen, A.; Asghar, Z.; Rehana, H. Coronavirus disease (COVID-19) and immunity booster green foods: A mini review. Food Sci. Nutr. 2020, 8, 3971–3976. [Google Scholar] [CrossRef]
- Rosanoff, A. Perspective: US adult magnesium requirements need updating: Impacts of rising body weights and data-derived variance. Adv. Nutr. 2021, 12, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Banjanin, N.; Belojevic, G. Relationship of dietary magnesium intake and serum magnesium with hypertension: A review. Magnes. Res. 2021, 34, 166–171. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: Double-blind, randomized clinical trial. Eur. J. Clin. Investig. 2011, 41, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Tayyem, R.; Al-Shudifat, A.E.; Al-Alami, Z.; Abdelbaset, M.G.; Al-Awwad, N.; Azab, M. Nutrition management in COVID-19 quarantine: Hospital-based study. Disaster Med. Public Health Prep. 2021, 17, e85. [Google Scholar] [CrossRef]
- Nouri-Majd, S.; Ebrahimzadeh, A.; Mousavi, S.M.; Zargarzadeh, N.; Eslami, M.; Santos, H.O.; Taghizadeh, M.; Milajerdi, A. Higher intake of dietary magnesium is inversely associated with COVID-19 severity and symptoms in hospitalized patients: A cross-sectional study. Front. Nutr. 2022, 9, 873162. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Shiraishi, M.; Harada, R.; Kurashima, Y. Association of lifestyle changes due to the COVID-19 pandemic with nutrient intake and physical activity levels during pregnancy in Japan. Nutrients 2021, 13, 3799. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Al-Khelefawi, Z.H.; Kalonarchi, G.; Papamikos, V. Formulation of the menu of a general hospital after its conversion to a “COVID hospital”: A nutrient analysis of 28-day menus. Front. Nutr. 2022, 9, 833628. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Eskander, M.; Razzaque, M.S. Can maintaining optimal magnesium balance reduce the disease severity of COVID-19 patients? Front. Endocrinol. 2022, 13, 843152. [Google Scholar] [CrossRef]
- Chacko, S.A.; Song, Y.; Nathan, L.; Tinker, L.; de Boer, I.H.; Tylavsky, F.; Wallace, R.; Liu, S. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010, 33, 304–310. [Google Scholar] [CrossRef]
- Ebrahimzadeh-Attari, V.; Panahi, G.; Hebert, J.R.; Ostadrahimi, A.; Saghafi-Asl, M.; Lotfi-Yaghin, N.; Baradaran, B. Nutritional approach for increasing public health during pandemic of COVID-19: A comprehensive review of antiviral nutrients and nutraceuticals. Health Promot. Perspect. 2021, 11, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Alfheeaid, H.A.; Rabbani, S.I. COVID-19: A review on the role of trace elements present in Saudi Arabian traditional dietary supplements. Pak. J. Biol. Sci. 2022, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Al-Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; Kaushik, D.; Verma, R.; Al-Kuraishy, H.M. Deciphering the immunoboosting potential of macro and micronutrients in COVID support therapy. Environ. Sci. Pollut. Res. Int. 2022, 29, 43516–43531. [Google Scholar] [CrossRef]
- Gozzi-Silva, S.C.; Teixeira, F.M.E.; Duarte, A.; Sato, M.N.; Oliveira, L.M. Immunomodulatory role of nutrients: How can pulmonary dysfunctions improve? Front. Nutr. 2021, 8, 674258. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Rapidly increasing serum 25(OH)D boosts the immune system, against infections-sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Kelishadi, R.; Ataei, E.; Ardalan, G.; Nazemian, M.; Tajadini, M.; Heshmat, R.; Keikha, M.; Motlagh, M.E. Relationship of serum magnesium and vitamin D levels in a nationally-representative sample of Iranian adolescents: The CASPIAN-III study. Int. J. Prev. Med. 2014, 5, 99–103. [Google Scholar]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin D activation and function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Rude, R.K.; Adams, J.S.; Ryzen, E.; Endres, D.B.; Niimi, H.; Horst, R.L.; Haddad, J.G., Jr.; Singer, F.R. Low serum concentrations of 1,25-dihydroxyvitamin D in human magnesium deficiency. J. Clin. Endocrinol. Metab. 1985, 61, 933–940. [Google Scholar] [CrossRef]
- Kisters, K.; Kisters, L.; Werner, T.; Deutsch, A.; Westhoff, T.; Gröber, U. Increased serum vitamin D concentration under oral magnesium therapy in elderly hypertensives. Magnes. Res. 2020, 33, 131–132. [Google Scholar] [CrossRef]
- Mursu, J.; Nurmi, T.; Voutilainen, S.; Tuomainen, T.P.; Virtanen, J.K. The association between serum 25-hydroxyvitamin D3 concentration and risk of disease death in men: Modification by magnesium intake. Eur. J. Epidemiol. 2015, 30, 343–347. [Google Scholar] [CrossRef]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B(12) in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79, 111017. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A. The high heart health value of drinking-water magnesium. Med. Hypotheses 2013, 81, 1063–1065. [Google Scholar] [CrossRef]
- Tian, J.; Tang, L.; Liu, X.; Li, Y.; Chen, J.; Huang, W.; Liu, M. Populations in low-magnesium areas were associated with higher risk of infection in COVID-19’s early transmission: A nationwide retrospective cohort study in the United States. Nutrients 2022, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, K.V.; Frolkov, V.K.; Nagornev, S.N.; Korchazhkina, N.B.; Gusakova, E.V.; Chelombitko, E.G. Prospects of drinking mineral water in the rehabilitation of patients with coronavirus (COVID-19) infection: Analysis of the main sanogenetic mechanisms. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2021, 98, 75–84. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Liu, J.; O’Keefe, J.H. Magnesium for the prevention and treatment of cardiovascular disease. Open. Heart 2018, 5, e000775. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Kumssa, D.B. Impact of rising body weight and cereal grain food processing on human magnesium nutrition. Plant Soil 2020, 457, 5–23. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Kumssa, D.B.; Broadley, M.R.; Watts, M.J.; Young, S.D.; Chilimba, A.D.C.; Ander, E.L. Dietary mineral supplies in Malawi: Spatial and socioeconomic assessment. BMC Nutr. 2015, 1, 42. [Google Scholar] [CrossRef]
- Tang, C.F.; Ding, H.; Jiao, R.Q.; Wu, X.X.; Kong, L.D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020, 886, 173546. [Google Scholar] [CrossRef]
- Simental-Mendia, L.E.; Sahebkar, A.; Rodriguez-Moran, M.; Zambrano-Galvan, G.; Guerrero-Romero, F. Effect of magnesium supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Curr. Pharm. Des. 2017, 23, 4678–4686. [Google Scholar] [CrossRef]
- Speakman, L.L.; Michienzi, S.M.; Badowski, M.E. Vitamins, supplements and COVID-19: A review of currently available evidence. Drugs Context. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Nabi-Afjadi, M.; Karami, H.; Goudarzi, K.; Alipourfard, I.; Bahreini, E. The effect of vitamin D, magnesium and zinc supplements on interferon signaling pathways and their relationship to control SARS-CoV-2 infection. Clin. Mol. Allergy 2021, 19, 21. [Google Scholar] [CrossRef]
- Faa, G.; Saba, L.; Fanni, D.; Kalcev, G.; Carta, M. Association between hypomagnesemia, COVID-19, respiratory tract and lung disease. Open Respir. Med. J. 2021, 15, 43–45. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Magnesium and vitamin D deficiency as a potential cause of immune dysfunction, cytokine storm and disseminated intravascular coagulation in COVID-19 patients. Mo. Med. 2021, 118, 68–73. [Google Scholar]
- Scarpati, G.; Baldassarre, D.; Oliva, F.; Pascale, G.; Piazza, O. Ionized or total magnesium levels, what should we measure in critical ill patients? Transl. Med. UniSa 2020, 23, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Wallace, T.C.; Butts, S.J.; Cao, S.; Ansu, V.; Spence, L.A.; Weaver, C.M.; Gletsu-Miller, N. Circulating ionized magnesium as a measure of supplement bioavailability: Results from a pilot study for randomized clinical trial. Nutrients 2020, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Jahnen, A.; Hesse, A. Bioavailability of magnesium from different pharmaceutical formulations. Urol. Res. 2011, 39, 123–127. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Important drug-micronutrient interactions: A selection for clinical practice. Crit. Rev. Food Sci. Nutr. 2020, 60, 257–275. [Google Scholar] [CrossRef]
- Walden, D.M.; Khotimchenko, M.; Hou, H.; Chakravarty, K.; Varshney, J. Effects of magnesium, calcium, and aluminum chelation on fluoroquinolone absorption rate and bioavailability: A computational study. Pharmaceutics 2021, 13, 594. [Google Scholar] [CrossRef]
- Santamarina, M.G.; Boisier, D.; Contreras, R.; Baque, M.; Volpacchio, M.; Beddings, I. COVID-19: A hypothesis regarding the ventilation-perfusion mismatch. Crit. Care 2020, 24, 395. [Google Scholar] [CrossRef]
- Nitsure, M.; Sarangi, B.; Shankar, G.H.; Reddy, V.S.; Walimbe, A.; Sharma, V.; Prayag, S. Mechanisms of hypoxia in COVID-19 patients: A pathophysiologic reflection. Indian J. Crit. Care Med. 2020, 24, 967–970. [Google Scholar] [CrossRef]
- Tipre, D.N.; Cidon, M.; Moats, R.A. Imaging pulmonary blood vessels and ventilation-perfusion mismatch in COVID-19. Mol. Imaging Biol. 2022, 24, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Mulia, E.P.B.; Luke, K. Inhaled prostacyclin analogues in COVID-19 associated acute respiratory distress syndrome: Scientific rationale. Egypt. Heart J. 2021, 73, 82. [Google Scholar] [CrossRef] [PubMed]
- Poonam, P.B.H.; Koscik, R.; Nguyen, T.; Rikhi, S.; Lin, H.M. Nitric oxide versus epoprostenol for refractory hypoxemia in COVID-19. PLoS ONE 2022, 17, e0270646. [Google Scholar] [CrossRef]
- Pourdowlat, G.; Mousavinasab, S.R.; Farzanegan, B.; Kashefizadeh, A.; Meybodi, Z.A.; Jafarzadeh, M.; Baniasadi, S. Evaluation of the efficacy and safety of inhaled magnesium sulphate in combination with standard treatment in patients with moderate or severe COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 60. [Google Scholar] [CrossRef]
- Pooransari, P.; Pourdowlat, G. Magnesium sulfate: A potential adjuvant treatment on COVID-19. Front. Emerg. Med. 2020, 5, e1. [Google Scholar]
- Shechter, M. The role of magnesium as antithrombotic therapy. Wien. Med. Wochenschr. 2000, 150, 343–347. [Google Scholar] [PubMed]
- Song, W.J.; Chang, Y.S. Magnesium sulfate for acute asthma in adults: A systematic literature review. Asia Pac. Allergy 2012, 2, 76–85. [Google Scholar] [CrossRef]
- Bolay, H.; Gül, A.; Baykan, B. COVID-19 is a real headache! Headache 2020, 60, 1415–1421. [Google Scholar] [CrossRef]
- Younes, B.; Alshawabkeh, A.D.; Jadallah, A.R.R.; Awwad, E.F.; Tarabsheh, T.M.I. Magnesium sulfate extended infusion as an adjunctive treatment for complicated COVID-19 infected critically ill patients. EAS J. Anesthesiol. Crit. Care 2020, 2, 97–101. [Google Scholar]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Simani, L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020, 413, 116832. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Giussani, G.; Westenberg, E.; Allegri, R.; Garcia-Azorin, D.; Guekht, A.; Frontera, J.; Kivipelto, M.; Mangialasche, F.; Mukaetova-Ladinska, E.B.; et al. Acute and post-acute neurological manifestations of COVID-19: Present findings, critical appraisal, and future directions. J. Neurol. 2022, 269, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ghosh, R.; Dubey, S.; Dubey, M.J.; Benito-León, J.; Kanti Ray, B. Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can. J. Neurol. Sci. 2021, 48, 9–24. [Google Scholar] [CrossRef]
- He, Y.; Bai, X.; Zhu, T.; Huang, J.; Zhang, H. What can the neurological manifestations of COVID-19 tell us: A meta-analysis. J. Transl. Med. 2021, 19, 363. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Pantelis, C.; Jayaram, M.; Hannan, A.J.; Wesselingh, R.; Nithianantharajah, J.; Wannan, C.M.; Syeda, W.T.; Choy, K.C.; Zantomio, D.; Christopoulos, A.; et al. Neurological, neuropsychiatric and neurodevelopmental complications of COVID-19. Aust. N. Z. J. Psychiatry 2021, 55, 750–762. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Filatov, A.; Sharma, P.; Hindi, F.; Espinosa, P.S. Neurological complications of coronavirus disease (COVID-19): Encephalopathy. Cureus 2020, 12, e7352. [Google Scholar] [CrossRef]
- Willison, H.J.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barré syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef]
- Shehata, G.A.; Lord, K.C.; Grudzinski, M.C.; Elsayed, M.; Abdelnaby, R.; Elshabrawy, H.A. Neurological complications of COVID-19: Underlying mechanisms and management. Int. J. Mol. Sci. 2021, 22, 4081. [Google Scholar] [CrossRef]
- van den Berg, B.; Walgaard, C.; Drenthen, J.; Fokke, C.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014, 10, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, K.A. Guillain-Barré syndrome. Continuum 2020, 26, 1184–1204. [Google Scholar] [CrossRef] [PubMed]
- Waddell, S.; Quinn, W.G. Flies, genes, and learning. Annu. Rev. Neurosci. 2001, 24, 1283–1309. [Google Scholar] [CrossRef] [PubMed]
- Baseler, H.A.; Aksoy, M.; Salawu, A.; Green, A.; Asghar, A.U.R. The negative impact of COVID-19 on working memory revealed using a rapid online quiz. PLoS ONE 2022, 17, e0269353. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Li, S.; Xu, R.; Nie, G.; Xie, Y.; Han, J.; Gao, X.; Zheng, Y.; Xu, Z.; Dai, Z. Post-COVID-19 human memory impairment: A PRISMA-based systematic review of evidence from brain imaging studies. Front. Aging Neurosci. 2022, 14, 1077384. [Google Scholar] [CrossRef]
- Durlach, J. Magnesium depletion and pathogenesis of Alzheimer’s disease. Magnes. Res. 1990, 3, 217–218. [Google Scholar]
- Chui, D.; Chen, Z.; Yu, J.; Zhang, H.; Wang, W.; Song, Y.; Yang, H.; Zhou, L. Magnesium in Alzheimer’s disease. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; pp. 239–250. [Google Scholar]
- Slutsky, I.; Abumaria, N.; Wu, L.J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.G.; Zhuo, M.; et al. Enhancement of learning and memory by elevating brain magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef]
- Won, J.; Silva, A.J. Molecular and cellular mechanisms of memory allocation in neuronetworks. Neurobiol. Learn. Mem. 2008, 89, 285–292. [Google Scholar] [CrossRef]
- Altura, B.M.; Kostellow, A.B.; Zhang, A.; Li, W.; Morrill, G.A.; Gupta, R.K.; Altura, B.T. Expression of the nuclear factor-kappaB and proto-oncogenes c-fos and c-jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: Possible links to hypertension, atherogenesis, and stroke. Am. J. Hypertens. 2003, 16, 701–707. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhou, X.; Liu, B.L.; Wei, X.H.; Ding, H.L.; Lin, Z.J.; Zhan, H.L.; Yang, F.; Li, W.B.; Xie, J.C.; et al. Normalization of magnesium deficiency attenuated mechanical allodynia, depressive-like behaviors, and memory deficits associated with cyclophosphamide-induced cystitis by inhibiting TNF-α/NF-κB signaling in female rats. J. Neuroinflamm. 2020, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Dean, O.; Bush, A.I.; Berk, M.; Copolov, D.L.; van den Buuse, M. Glutathione depletion in the brain disrupts short-term spatial memory in the Y-maze in rats and mice. Behav. Brain Res. 2009, 198, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Mayford, M.; Siegelbaum, S.A.; Kandel, E.R. Synapses and memory storage. Cold Spring Harb. Perspect. Biol. 2012, 4, a005751. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, I.; Sadeghpour, S.; Li, B.; Liu, G. Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. Neuron 2004, 44, 835–849. [Google Scholar] [CrossRef]
- Suzuki, A.; Fukushima, H.; Mukawa, T.; Toyoda, H.; Wu, L.J.; Zhao, M.G.; Xu, H.; Shang, Y.; Endoh, K.; Iwamoto, T.; et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J. Neurosci. 2011, 31, 8786–8802. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Zhang, L.; Han, F.; Pang, K.L.; Li, X.; Shen, J.Y. Magnesium boosts the memory restorative effect of environmental enrichment in Alzheimer’s disease mice. CNS Neurosci. Ther. 2018, 24, 70–79. [Google Scholar] [CrossRef]
- Taghizadeh-Hesary, F.; Akbari, H. The powerful immune system against powerful COVID-19: A hypothesis. Med. Hypotheses 2020, 140, 109762. [Google Scholar] [CrossRef]
- Weglicki, W.B.; Phillips, T.M.; Mak, I.T.; Cassidy, M.M.; Dickens, B.F.; Stafford, R.; Kramer, J.H. Cytokines, neuropeptides, and reperfusion injury during magnesium deficiency. Ann. N. Y. Acad. Sci. 1994, 723, 246–257. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and memory processing. Neuropharmacology 2014, 76, 677–683. [Google Scholar] [CrossRef]
- Abiri, B.; Sarbakhsh, P.; Vafa, M. Randomized study of the effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammation, and SIRT1 in obese women with mild to moderate depressive symptoms. Nutr. Neurosci. 2022, 25, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.F.; Xue, X.Y.; Liao, M.J.; Xiao, F.; Lv, R.H.; Luo, H.M. Neurotrophic effects of magnesium fructose 1, 6-diphosphate on cortical neurons. Int. J. Neurosci. 2012, 122, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Serita, T.; Miyahara, M.; Tanimizu, T.; Takahashi, S.; Oishi, S.; Nagayoshi, T.; Tsuji, R.; Inoue, H.; Uehara, M.; Kida, S. Dietary magnesium deficiency impairs hippocampus-dependent memories without changes in the spine density and morphology of hippocampal neurons in mice. Brain Res. Bull. 2019, 144, 149–157. [Google Scholar] [CrossRef]
- Wang, Y.; Zajac, A.L.; Lei, W.; Christensen, C.M.; Margolskee, R.F.; Bouysset, C.; Golebiowski, J.; Zhao, H.; Fiorucci, S.; Jiang, P. Metal ions activate the human taste receptor TAS2R7. Chem. Senses 2019, 44, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Salzano, G.; Deiana, G.; De Riu, G. Anosmia and ageusia: Common findings in COVID-19 patients. Laryngoscope 2020, 130, 1787. [Google Scholar] [CrossRef]
- Henkin, R.I. Drug-induced taste and smell disorders. Incidence, mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf. 1994, 11, 318–377. [Google Scholar] [CrossRef]
- Xydakis, M.S.; Dehgani-Mobaraki, P.; Holbrook, E.H.; Geisthoff, U.W.; Bauer, C.; Hautefort, C.; Herman, P.; Manley, G.T.; Lyon, D.M.; Hopkins, C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020, 20, 1015–1016. [Google Scholar] [CrossRef]
- McCaughey, S.A.; Tordoff, M.G. Magnesium appetite in the rat. Appetite 2002, 38, 29–38. [Google Scholar] [CrossRef]
- Barrea, L.; Grant, W.B.; Frias-Toral, E.; Vetrani, C.; Verde, L.; de Alteriis, G.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Dietary recommendations for post-COVID-19 syndrome. Nutrients 2022, 14, 1305. [Google Scholar] [CrossRef]
- Kumar, S.S.; Khushbu, G.; Dev, M.J. Hypomagnesaemia induced recurrent cerebellar ataxia: An interesting case with successful management. Cerebellum Ataxias 2020, 7, 1. [Google Scholar] [CrossRef]
- Blasco, L.M. Cerebellar syndrome in chronic cyclic magnesium depletion. Cerebellum 2013, 12, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Boulos, M.I.; Shoamanesh, A.; Aviv, R.I.; Gladstone, D.J.; Swartz, R.H. Severe hypomagnesemia associated with reversible subacute ataxia and cerebellar hyperintensities on MRI. Neurologist 2012, 18, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Te Riele, M.G.; Verrips, A. Severe hypomagnesaemia causing reversible cerebellopathy. Cerebellum 2014, 13, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Almoussa, M.; Goertzen, A.; Brauckmann, S.; Fauser, B.; Zimmermann, C.W. Posterior reversible encephalopathy syndrome due to hypomagnesemia: A case report and literature review. Case Rep. Med. 2018, 2018, 1980638. [Google Scholar] [CrossRef] [PubMed]
- Caplan, J.P.; Chang, G. Refeeding syndrome as an iatrogenic cause of delirium: A retrospective pilot study. Psychosomatics 2010, 51, 419–424. [Google Scholar] [CrossRef]
- Isse, N.; Hashimoto, M. Omeprazole-induced hypomagnesaemia, causing renal tubular acidosis with hypokalaemia, hypocalcaemia, hyperlactacidaemia and hyperammonaemia. BMJ Case Rep. 2020, 13, e235385. [Google Scholar] [CrossRef]
- Horino, T.; Ichii, O.; Terada, Y. A rare presentation of hypermagnesemia associated with acute kidney injury due to hypercalcemia. Intern. Med. 2019, 58, 1123–1126. [Google Scholar] [CrossRef]
- Devita, M.; Bordignon, A.; Sergi, G.; Coin, A. The psychological and cognitive impact of COVID-19 on individuals with neurocognitive impairments: Research topics and remote intervention proposals. Aging Clin. Exp. Res. 2021, 33, 733–736. [Google Scholar] [CrossRef]
- Hassett, C.E.; Gedansky, A.; Migdady, I.; Bhimraj, A.; Uchino, K.; Cho, S.M. Neurologic complications of COVID-19. Cleve Clin. J. Med. 2020, 87, 729–734. [Google Scholar] [CrossRef]
- Baker, S.B.; Worthley, L.I. The essentials of calcium, magnesium and phosphate metabolism: Part II. Disorders. Crit. Care Resusc. 2002, 4, 307–315. [Google Scholar]
- Gutiérrez-Ortiz, C.; Méndez-Guerrero, A.; Rodrigo-Rey, S.; San Pedro-Murillo, E.; Bermejo-Guerrero, L.; Gordo-Mañas, R.; de Aragón-Gómez, F.; Benito-León, J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020, 95, e601–e605. [Google Scholar] [CrossRef]
- Cavalagli, A.; Peiti, G.; Conti, C.; Penati, R.; Vavassori, F.; Taveggia, G. Cranial nerves impairment in post-acute oropharyngeal dysphagia after COVID-19. Eur. J. Phys. Rehabil. Med. 2020, 56, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Ponfick, M.; Linden, R.; Nowak, D.A. Dysphagia—A common, transient symptom in critical illness polyneuropathy: A fiberoptic endoscopic evaluation of swallowing study. Crit. Care Med. 2015, 43, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Decavel, P.; Petit, C.; Tatu, L. Tapia syndrome at the time of the COVID-19 pandemic: Lower cranial neuropathy following prolonged intubation. Neurology 2020, 95, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Sajed, S. Seizures related to coronavirus disease (COVID-19): Case series and literature review. Cureus 2020, 12, e9378. [Google Scholar] [CrossRef] [PubMed]
- Codadu, N.K.; Graham, R.T.; Burman, R.J.; Jackson-Taylor, R.T.; Raimondo, J.V.; Trevelyan, A.J.; Parrish, R.R. Divergent paths to seizure-like events. Physiol. Rep. 2019, 7, e14226. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.G.; Calleja, S.; Suarez, L.; Pascual, J. Recurrent confusional episodes associated with hypomagnesaemia due to esomeprazol. BMJ Case Rep. 2013, 2013, bcr2013200501. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Barrera, J.; Urdiroz, J.; Centeno, C. Visual hallucinations and unusual pain related to hypomagnesemia in an advanced cancer patient. An. Sist. Sanit. Navar. 2010, 33, 319–322. [Google Scholar] [CrossRef]
- Kuramoto, T.; Kuwamura, M.; Tokuda, S.; Izawa, T.; Nakane, Y.; Kitada, K.; Akao, M.; Guénet, J.L.; Serikawa, T. A mutation in the gene encoding mitochondrial Mg2+ channel MRS2 results in demyelination in the rat. PLoS Genet. 2011, 7, e1001262. [Google Scholar] [CrossRef]
- Yasui, M.; Ota, K. Experimental and clinical studies on dysregulation of magnesium metabolism and the aetiopathogenesis of multiple sclerosis. Magnes. Res. 1992, 5, 295–302. [Google Scholar]
- Haji Akhoundi, F.; Sahraian, M.A.; Naser Moghadasi, A. Neuropsychiatric and cognitive effects of the COVID-19 outbreak on multiple sclerosis patients. Mult. Scler. Relat. Disord. 2020, 41, 102164. [Google Scholar] [CrossRef]
- Yasui, M.; Yase, Y.; Ando, K.; Adachi, K.; Mukoyama, M.; Ohsugi, K. Magnesium concentration in brains from multiple sclerosis patients. Acta Neurol. Scand. 1990, 81, 197–200. [Google Scholar] [CrossRef]
- Goldberg, P.; Fleming, M.C.; Picard, E.H. Multiple sclerosis: Decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med. Hypotheses 1986, 21, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Visconti, A.; Santucci, S.; Ghazaryan, A.; Figà-Talamanca, L.; Cannoni, S.; Bocca, B.; Pino, A.; Violante, N.; Alimonti, A.; et al. Quantification of chemical elements in blood of patients affected by multiple sclerosis. Ann. Ist. Super. Sanita 2005, 41, 213–216. [Google Scholar] [PubMed]
- de Oliveira, M.; Gianeti, T.M.R.; da Rocha, F.C.G.; Lisboa-Filho, P.N.; Piacenti-Silva, M. A preliminary study of the concentration of metallic elements in the blood of patients with multiple sclerosis as measured by ICP-MS. Sci. Rep. 2020, 10, 13112. [Google Scholar] [CrossRef]
- Bobker, S.M.; Robbins, M.S. COVID-19 and headache: A primer for trainees. Headache 2020, 60, 1806–1811. [Google Scholar] [CrossRef]
- Caronna, E.; Pozo-Rosich, P. Headache as a symptom of COVID-19: Narrative review of 1-year research. Curr. Pain Headache Rep. 2021, 25, 73. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Edvinsson, L. Neurogenic inflammation: A study of rat trigeminal ganglion. J. Headache Pain 2010, 11, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.S.; Singh, J.; Lyall, J.S. A new horizon into the pathobiology, etiology and treatment of migraine. Med. Hypotheses 2011, 77, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Delsere, M.; Campogiani, V.; Carletti, V.; Mancini, S.; Piccinini, N.; Castelli, P.; Sopranzi, F. Epilepsy, vertigo, dizziness, headache, emesis as neurological manifestation in a Giteleman’s Sindrome case. G. Ital. Nefrol. 2015, 32, gin/32.36.13. [Google Scholar]
- Santinelli, V.; Ferraiuolo, M.; Modano, P.; Oppo, I.; Chiariello, M.; Fornaro, P. Magnesium deficiency and dizziness: A case of electrolyte imbalance. Geriatrics 1999, 54, 67–68, 73. [Google Scholar]
- Micke, O.; Vormann, J.; Kisters, K. Magnesium and COVID-19: Some further comments—A commentary on Wallace TC. Combating COVID-19 and building immune resilience: A potential role for magnesium nutrition? J. Am. Coll. Nutr. 2021, 40, 732–734. [Google Scholar] [CrossRef]
- Micke, O.; Vormann, J.; Kisters, K. Magnesium deficiency and COVID-19—What are the links? Some remarks from the German society for magnesium research. Trace Elem. Electrolytes 2020, 37, 103–107. [Google Scholar] [CrossRef]
- Drożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybyciński, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Łos, M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updat. 2021, 59, 100794. [Google Scholar] [CrossRef] [PubMed]
- Knorr, J.P.; Colomy, V.; Mauriello, C.M.; Ha, S. Tocilizumab in patients with severe COVID-19: A single-center observational analysis. J. Med. Virol. 2020, 92, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Painter, W.P.; Holman, W.; Bush, J.A.; Almazedi, F.; Malik, H.; Eraut, N.; Morin, M.J.; Szewczyk, L.J.; Painter, G.R. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, e02428-20. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Honoré, S.; Hoang, V.T.; Raoult, D. Clinical efficacy and safety profile of hydroxychloroquine and azithromycin against COVID-19. Int. J. Antimicrob. Agents 2021, 57, 106242. [Google Scholar] [CrossRef] [PubMed]
- Sada, M.; Saraya, T.; Ishii, H.; Okayama, K.; Hayashi, Y.; Tsugawa, T.; Nishina, A.; Murakami, K.; Kuroda, M.; Ryo, A.; et al. Detailed molecular interactions of favipiravir with SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza virus polymerases in silico. Microorganisms 2020, 8, 1610. [Google Scholar] [CrossRef]
- Duksal, F.; Burnik, C.; Mermer, M.; Yavuz, S. Evaluation of the effect of biochemistry parameters on the clinical course in COVID-19 patients who received tocilizumab treatment. South. Med. J. 2022, 115, 435–440. [Google Scholar] [CrossRef]
- Lippi, G.; South, A.M.; Henry, B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann. Clin. Biochem. 2020, 57, 262–265. [Google Scholar] [CrossRef]
- Moon, J.; Cho, E.S.; Lee, M.Y.; Son, H.Y.; Lee, K. Magnesium augments immunosuppressive effects of a corticosteroid in obese mice with airway inflammation. Asian Pac. J. Allergy Immunol. 2021, 39, 15–24. [Google Scholar] [CrossRef]
- Lutgendorf, M.A.; Ippolito, D.L.; Mesngon, M.T.; Tinnemore, D.; Dehart, M.J.; Dolinsky, B.M.; Napolitano, P.G. Effect of dexamethasone administered with magnesium sulfate on inflammation-mediated degradation of the blood-brain barrier using an in vitro model. Reprod. Sci. 2014, 21, 483–491. [Google Scholar] [CrossRef]
- Elalfy, H.; Besheer, T.; El-Mesery, A.; El-Gilany, A.H.; Soliman, M.A.; Alhawarey, A.; Alegezy, M.; Elhadidy, T.; Hewidy, A.A.; Zaghloul, H.; et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J. Med. Virol. 2021, 93, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Ashraf, S.; Farooq, I.; Ashraf, S.; Ashraf, M.; Imran, M.A.; Kalsoom, L.; Akmal, R.; Ghufran, M.; Rafique, S.; et al. Anti-COVID property of subcutaneous ivermectin in synergy with zinc among midlife moderately symptomatic patients: A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 591. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Samman, S. Zinc and regulation of inflammatory cytokines: Implications for cardiometabolic disease. Nutrients 2012, 4, 676–694. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.K.; Smyth, M.J.; Stennicke, H.R.; Salvesen, G.S.; Duriez, P.; Poirier, G.G.; Hannun, Y.A. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J. Biol. Chem. 1997, 272, 18530–18533. [Google Scholar] [CrossRef]
- Frontera, J.A.; Rahimian, J.O.; Yaghi, S.; Liu, M.; Lewis, A.; de Havenon, A.; Mainali, S.; Huang, J.; Scher, E.; Wisniewski, T.; et al. Treatment with Zinc is Associated with Reduced In-Hospital Mortality among COVID-19 Patients: A Multi-Center Cohort Study (Preprint). Res. Sq. 2020. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC7605567/pdf/nihpp-rs94509v1.pdf (accessed on 1 November 2022).
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020, 69, 1228–1234. [Google Scholar] [CrossRef]
- Jandaghi, P.; Hosseini, Z.; Chilibeck, P.; Hanley, A.J.; Deguire, J.R.; Bandy, B.; Pahwa, P.; Vatanparast, H. The role of immunomodulatory nutrients in alleviating complications related to SARS-CoV-2: A scoping review. Adv. Nutr. 2021, 13, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Derwand, R.; Scholz, M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Med. Hypotheses 2020, 142, 109815. [Google Scholar] [CrossRef]
- te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Mannuß, S.; Schuff-Werner, P.; Dreißiger, K.; Kohlschein, P. Magnesium sulfate as an alternative in vitro anticoagulant for the measurement of platelet parameters? Am. J. Clin. Pathol. 2016, 145, 806–814. [Google Scholar] [CrossRef] [PubMed]
- James, M.F.; Neil, G. Effect of magnesium on coagulation as measured by thrombelastography. Br. J. Anaesth. 1995, 74, 92–94. [Google Scholar] [CrossRef]
- Mese, K.; Bunz, O.; Volkwein, W.; Vemulapalli, S.P.B.; Zhang, W.; Schellhorn, S.; Heenemann, K.; Rueckner, A.; Sing, A.; Vahlenkamp, T.W.; et al. Enhanced antiviral function of magnesium chloride-modified heparin on a broad spectrum of viruses. Int. J. Mol. Sci. 2021, 22, 10075. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sugatani, J.; Ino, M.; Shimura, M.; Akiyama, M.; Yamazaki, R.; Suzuki, Y.; Miwa, M. Continuous binding of the PAF molecule to its receptor is necessary for the long-term aggregation of platelets. Am. J. Physiol. 1998, 274, C47–C57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shields, L.B.E.; Gao, Z.; Wang, Y.; Zhang, Y.P.; Chu, T.; Zhu, Q.; Shields, C.B.; Cai, J. Current understanding of platelet-activating factor signaling in central nervous system diseases. Mol. Neurobiol. 2017, 54, 5563–5572. [Google Scholar] [CrossRef]
- Vargaftig, B.B.; Lefort, J.; Chignard, M.; Benveniste, J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur. J. Pharmacol. 1980, 65, 185–192. [Google Scholar] [CrossRef]
- Costuleanu, M.; Brailoiu, E.; Filipeanu, C.M.; Baltatu, O.; Slatineanu, S.; Saila, L.; Nechifor, M.; Branisteanu, D.D. Effects of liposome-entrapped platelet-activating factor in the isolated rat trachea. Eur. J. Pharmacol. 1995, 281, 89–92. [Google Scholar] [CrossRef]
- Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, phytochemicals and Mediterranean diet: A potential protective role against COVID-19 through modulation of PAF actions and metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef]
- Evangelou, A.; Kalfakakou, V.; Benveniste, J.; Arnoux, B. Inhibition of PAF-acether effects on isolated guinea pig hearts by zinc ions (Zn2+). Biol. Trace Elem. Res. 1995, 50, 43–55. [Google Scholar] [CrossRef]
- Nechifor, M.; Neughebauer, B.I.; Adomnicĭ, M.; Teslaru, E.; Filip, C.; Negru, A. The influence of some cations on PAF-induced gastric mucosal damage in rats. Rom. J. Physiol. 1993, 30, 151–154. [Google Scholar]
- Kawaguchi, H.; Sawa, H.; Yasuda, H. Mechanism of increased angiotensin-converting enzyme activity stimulated by platelet-activating factor. Biochim. Biophys. Acta 1990, 1052, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wang, H.; Chen, Z.; Liu, Q. Context contribution to the intermolecular recognition of human ACE2-derived peptides by SARS-CoV-2 spike protein: Implications for improving the peptide affinity but not altering the peptide specificity by optimizing indirect readout. Mol. Omics 2021, 17, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, B.; Hashimoto, K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav. Immun. 2020, 87, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Salgo, M.P. COVID-19: Zinc and angiotensin-converting enzyme 2 (ACE2) deficiencies as determinants of risk and severity of disease: A narrative review. Infect. Dis. Ther. 2021, 10, 1215–1225. [Google Scholar] [CrossRef]
- Hall, K.; Mfone, F.; Shallcross, M.; Pathak, V. Review of pharmacotherapy trialed for management of the coronavirus disease-19. Eurasian J. Med. 2021, 53, 137–143. [Google Scholar] [CrossRef]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Papakonstantinou, V.D.; Lagopati, N.; Tsilibary, E.C.; Demopoulos, C.A.; Philippopoulos, A.I. A review on platelet activating factor inhibitors: Could a new class of potent metal-based anti-inflammatory drugs induce anticancer properties? Bioinorg. Chem. Appl. 2017, 2017, 6947034. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Braun, A. Zinc homeostasis in platelet-related diseases. Int. J. Mol. Sci. 2019, 20, 5258. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef]
- Ebel, H.; Günther, T. Stimulation of choline/Mg2+ antiport in rat erythrocytes by mefloquine. Magnes. Res. 2006, 19, 7–11. [Google Scholar] [PubMed]
- Rahman, M.T.; Idid, S.Z. Can Zn be a critical element in COVID-19 treatment? Biol. Trace Elem. Res. 2021, 199, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Asakawa, A.; Ueda, H.; Ikeda, S.; Miyawaki, S.; Inui, A. The role of zinc in the treatment of taste disorders. Recent. Pat. Food Nutr. Agric. 2013, 5, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Roussel, A.M.; Zouari, N.; Mahjoub, S.; Matheau, J.M.; Kerkeni, A. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J. Am. Coll. Nutr. 2001, 20, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Ervin, R.B.; Kennedy-Stephenson, J. Mineral intakes of elderly adult supplement and non-supplement users in the Third National Health and Nutrition Examination Survey. J. Nutr. 2002, 132, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447s–463s. [Google Scholar] [CrossRef]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452s–1456s. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of current clinical trials. Biol. Trace Elem. Res. 2021, 199, 2882–2892. [Google Scholar] [CrossRef]
- Singh, R.; Shaik, L.; Mehra, I.; Kashyap, R.; Surani, S. Novel and controversial therapies in COVID-19. Open Respir. Med. J. 2020, 14, 79–86. [Google Scholar] [CrossRef]
- Kumar, A.; Kubota, Y.; Chernov, M.; Kasuya, H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses 2020, 144, 109848. [Google Scholar] [CrossRef] [PubMed]
- Foulds, G.; Hilligoss, D.M.; Henry, E.B.; Gerber, N. The effects of an antacid or cimetidine on the serum concentrations of azithromycin. J. Clin. Pharmacol. 1991, 31, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Stass, H.; Böttcher, M.F.; Ochmann, K. Evaluation of the influence of antacids and H2 antagonists on the absorption of moxifloxacin after oral administration of a 400 mg dose to healthy volunteers. Clin. Pharmacokinet. 2001, 40 (Suppl. S1), 39–48. [Google Scholar] [CrossRef]
- Naggar, V.F.; Khalil, S.A.; Gouda, M.W. Effect of concomitant administration of magnesium trisilicate on GI absorption of dexamethasone in humans. J. Pharm. Sci. 1978, 67, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.; Hannafon, B.N.; Ding, W.Q. Chloroquine is a zinc ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef]
- Chary, M.A.; Barbuto, A.F.; Izadmehr, S.; Hayes, B.D.; Burns, M.M. COVID-19: Therapeutics and their toxicities. J. Med. Toxicol. 2020, 16, 284–294. [Google Scholar] [CrossRef]
- Carron, J.; Sharif, Z.; Hussein, H.; Kennedy, M.; McAdam, B.; Sheahan, R. Clinical guidance for navigating the QTc-prolonging and arrhythmogenic potential of pharmacotherapy during the COVID-19 pandemic. Ir. J. Med. Sci. 2021, 190, 403–409. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19). Mayo Clin. Proc. 2020, 95, 1213–1221. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, F.L.; Lin, C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin. Toxicol. 2006, 44, 173–175. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Ackerman, M.J. Azithromycin and risk of sudden cardiac death: Guilty as charged or falsely accused? Cleve Clin. J. Med. 2013, 80, 539–544. [Google Scholar] [CrossRef]
- Arellano-Rodrigo, E.; García, A.; Mont, L.; Roqué, M. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome. Med. Clin. 2001, 117, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Beyls, C.; Martin, N.; Hermida, A.; Abou-Arab, O.; Mahjoub, Y. Lopinavir-ritonavir treatment for COVID-19 infection in intensive care unit: Risk of bradycardia. Circ. Arrhythm. Electrophysiol. 2020, 13, e008798. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male 2020, 23, 1416–1424. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; He, K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: Meta-analysis and systematic review. Eur. J. Clin. Nutr. 2014, 68, 510–516. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Ellis, T. Magnesium intake and serum C-reactive protein levels in children. Magnes. Res. 2007, 20, 32–36. [Google Scholar] [PubMed]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef]
- Makwana, S.; Patel, A.; Sonagara, M. Correlation between serum magnesium level and acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). Cureus 2022, 14, e26229. [Google Scholar] [CrossRef]
- do Amaral, A.F.; Rodrigues-Júnior, A.L.; Terra Filho, J.; Vannucchi, H.; Martinez, J.A. Effects of acute magnesium loading on pulmonary function of stable COPD patients. Med. Sci. Monit. 2008, 14, Cr524–Cr529. [Google Scholar]
- Zanforlini, B.M.; Ceolin, C.; Trevisan, C.; Alessi, A.; Seccia, D.M.; Noale, M.; Maggi, S.; Guarnieri, G.; Vianello, A.; Sergi, G. Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin. Exp. Res. 2022, 34, 167–174. [Google Scholar] [CrossRef]
- Pourfridoni, M.; Abbasnia, S.M.; Shafaei, F.; Razaviyan, J.; Heidari-Soureshjani, R. Fluid and electrolyte disturbances in COVID-19 and their complications. BioMed Res. Int. 2021, 2021, 6667047. [Google Scholar] [CrossRef]
- Song, H.; Chia, A.Z.Q.; Tan, B.K.J.; Teo, C.B.; Lim, V.; Chua, H.R.; Samuel, M.; Kee, A. Electrolyte imbalances as poor prognostic markers in COVID-19: A systemic review and meta-analysis. J. Endocrinol. Investig. 2023, 46, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.M.; Silva, N.; Moura, A.F.; Duarte Silveira, M.A.; Moura-Neto, J.A. Acute kidney injury and electrolyte disorders in COVID-19. World J. Virol. 2022, 11, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Whang, R.; Oei, T.O.; Aikawa, J.K.; Watanabe, A.; Vannatta, J.; Fryer, A.; Markanich, M. Predictors of clinical hypomagnesemia. Hypokalemia, hypophosphatemia, hyponatremia, and hypocalcemia. Arch. Intern. Med. 1984, 144, 1794–1796. [Google Scholar] [CrossRef] [PubMed]
- Yasari, F.; Akbarian, M.; Abedini, A.; Vasheghani, M. The role of electrolyte imbalances in predicting the severity of COVID-19 in the hospitalized patients: A cross-sectional study. Sci. Rep. 2022, 12, 14732. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; West, C.; Elin, R.J.; Micke, O.; Baniasadi, S.; Barbagallo, M.; Campbell, E.; Cheng, F.C.; Costello, R.B.; Gamboa-Gomez, C.; et al. Recommendation on an updated standardization of serum magnesium reference ranges. Eur. J. Nutr. 2022, 61, 3697–3706. [Google Scholar] [CrossRef]

| Mg Status | Serum Mg Level | COVID-19–Associated Outcomes | ||
|---|---|---|---|---|
| mg/dL | mmol/L | mEq/L | ||
| Severe hypomagnesemia | <1.58 | <0.65 | <1.3 | Present in 13% of hospitalized patients with COVID-19 [52] |
| Hypomagnesemia | <2.0 | <0.82 | <1.65 | Risk factor for severe COVID-19 [54] |
| <1.82 | <0.75 | <1.5 | Defines Mg deficiency; increased length of CCU stay [52] | |
| 1.80 | 0.74 | 1.48 | Infection severity and worsened prognosis in ICU patients with COVID-19 [55] | |
| ≤1.8 | <0.74 | <1.48 | Death [53]; predicts arterial thromboembolism [56] | |
| High normal | 2.19–2.26 | 0.90–0.93 | 1.8–1.86 | Protective against deterioration [54] |
| Pulmonary/Respiratory Complication of COVID-19 | Possible Link with Mg |
|---|---|
| Respiratory system is the main organ system involved in COVID-19 [130] | Intravenous Mg sulfate has a clinical impact on acute severe asthma [129] |
| Mg sulfate can dilate constricted pulmonary arteries and reduce pulmonary artery resistance as well as induce bronchodilation by inhibition of airway smooth muscle contraction [126] | |
| Mg sulfate extended infusion has been suggested as an adjunctive treatment for critically ill patients with COVID-19 [131] | |
| V/Q mismatch in COVID-19 may be responsible for severe respiratory complications of COVID-19 [121,122,123] | |
| Inhalation therapies for COVID-19 suggested to improve oxygenation and V/Q mismatch [124,125] | Nebulized Mg sulfate therapy has been proposed to reduce V/Q mismatch and improve oxygenation [126] |
| Nebulized Mg sulfate improved oxygenation in patients with severe COVID-19 with hypoxia with V/Q mismatch but less so for critically and very severely ill patients with COVID-19 with some degree of intrapulmonary shunt [126] [manuscript in preparation] |
| Neurological or Psychiatric Complication of COVID-19 | Possible Link with Mg |
|---|---|
| Memory and cognition [147,148] | Mg plays a role in mechanisms of memory and cognition, and Mg deficit is involved in significant reduction in memory [149,150,159] |
| Increasing brain Mg in rats is positively correlated with increased working, long-term, and short-term memory [151] | |
| Mg has been shown to be involved in prevention of memory loss or recovery of memory via | |
| Impaired c-Fos activation [152] | Impaired activation of c-Fos is necessary for memory formation [152] |
| Low Mg impairs c-Fos activity [153] | |
| Mg administration normalizes c-Fos expression [153] | |
| Neuroplasticity and synaptic interconnection mediate memory [156] | Mg improves synaptic plasticity [151] |
| Mg plus environmental enrichment synergistically improved recognition/spatial memory by reducing synaptic loss and restoring the NMDAR signaling pathway in AD mice [159] | |
| Chronically reducing Ca2+ flux enhancement synaptic plasticity [151] | |
| CREB-mediated transcription involved in long-and short-term memory [152] | Mg upregulated CREB-mediated transcription [158] |
| Loss of taste and smell [138,143,170] | TAS2R7 taste receptor is activated by Mg [167] |
| Patients with COVID-19 and low Mg status showed change and/or loss of taste and/or smell [168,170] | |
| Loss of appetite [85,130,172] | Mg deficiency causes loss of appetite [171] |
| Ataxia [138] | Mg depletion is associated with decreased cerebellar functions, including ataxia [24,173,174,175,176,177] |
| Mg administration contributed to improvement in cerebellar clinical symptoms [173,174,175] | |
| There are no clinical trials of Mg therapy for patients with patients with COVID-19 presenting with ataxia, but we consider Mg administration should be considered and studied | |
| Impaired consciousness [132,138] | Impaired consciousness occurs in about 14% of patients with patients with COVID-19 [132,138] |
| Both hypo- and hyper-magnesemia sometimes cause severe disturbances of consciousness [179,180] | |
| Mental fatigue and inattention [181,182] | These clinical manifestations were reversible with Mg administration [183] |
| Demyelination, cranial nerve palsy, and axonal neuropathies [184] | In patients with MS, Mg has protective action at the level of myelin [193,194,195,196]. MS demyelination is associated with low plasma as well as low cerebral spinal fluid Mg [197]; these low blood Mg levels are associated with decreased concentrations of other metallic elements [198] |
| Data on Mg and metal trace elements in patients with patients with COVID-19 are few, but we consider that partial protective mechanism of Mg in MS is the same mechanism by which Mg can reduce demyelination in COVID-19 complications | |
| Convulsions or generalized seizures [138,143,188] | Hypomagnesemia is associated with the production of convulsions, lower convulsive threshold, and increases in brain glutamate concentrations [25,183] |
| Headache [130,138,143,199] | Headache complication of COVID-19 related to direct viral invasion as well as cytokine release syndrome [130,199] and hypoxia [130], both of which are associated with low Mg status [115,126] |
| Dizziness [137,138,143] | Mg administration improves symptoms and alleviates dizziness [203,204] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Romero, F.; Micke, O.; Simental-Mendía, L.E.; Rodríguez-Morán, M.; Vormann, J.; Iotti, S.; Banjanin, N.; Rosanoff, A.; Baniasadi, S.; Pourdowlat, G.; et al. Importance of Magnesium Status in COVID-19. Biology 2023, 12, 735. https://0-doi-org.brum.beds.ac.uk/10.3390/biology12050735
Guerrero-Romero F, Micke O, Simental-Mendía LE, Rodríguez-Morán M, Vormann J, Iotti S, Banjanin N, Rosanoff A, Baniasadi S, Pourdowlat G, et al. Importance of Magnesium Status in COVID-19. Biology. 2023; 12(5):735. https://0-doi-org.brum.beds.ac.uk/10.3390/biology12050735
Chicago/Turabian StyleGuerrero-Romero, Fernando, Oliver Micke, Luis E. Simental-Mendía, Martha Rodríguez-Morán, Juergen Vormann, Stefano Iotti, Nikolina Banjanin, Andrea Rosanoff, Shadi Baniasadi, Guitti Pourdowlat, and et al. 2023. "Importance of Magnesium Status in COVID-19" Biology 12, no. 5: 735. https://0-doi-org.brum.beds.ac.uk/10.3390/biology12050735