Metal Extraction Processes for Electronic Waste and Existing Industrial Routes: A Review and Australian Perspective
Abstract
:1. Introduction
1.1. Definitions, Classification and Composition of E-Waste
European WEEE Directive“Electrical or electronic equipment which is waste … including all components, sub-assemblies and consumables, which are part of the product at the time of discarding.”
Basel Action Network“E-waste encompasses a broad and growing range of electronic devices ranging from large household devices such as refrigerators, air conditioners, cell phones, personal stereos, and consumers electronics to computers which have been discarded by their users.”[4]
| No. | Category | Label |
|---|---|---|
| 1 | Large household appliances | Large HH |
| 2 | Small household appliances | Small HH |
| 3 | IT and telecommunications equipment | ICT |
| 4 | Consumer equipment | CE |
| 5 | Lighting equipment | Lighting |
| 6 | Electrical and electronic tools (with the exceptions of large-scale stationary industrial tools) | E & E tools |
| 7 | Toys, leisure and sport equipment | Toys |
| 8 | Medical devices (with the exception of all implanted and infected products) | Medical equipment |
| 9 | Monitoring and control instruments | M & C |
| 10 | Automatic dispensers | Dispensers |
| No. | Material | Percentage |
|---|---|---|
| 1 | Ferrous | 38 |
| 2 | Non-ferrous | 28 |
| 3 | Plastics | 19 |
| 4 | Glass | 4 |
| 5 | Wood | 1 |
| 6 | Other | 10 |
| Materials | Typical Concentrations (in wt% and ppm) | |||
|---|---|---|---|---|
| Metals (Max. wt. 40%) | Shuey and Taylor [16] | Kim et al. [17] | Iji and Yokoyama [18] | Ewasteguide.info [19] |
| Cu | 20 | 15.6 | 22 | 6.9 |
| Al | 2 | - | - | 14.2 |
| Pb | 2 | 1.35 | 1.55 | 6.3 |
| Zn | 1 | 0.16 | - | 2.2 |
| Ni | 2 | 0.28 | 0.32 | 0.85 |
| Fe | 8 | 1.4 | 3.6 | 20.5 |
| Sn | 4 | 3.24 | 2.6 | 1.0 |
| Sb/ppm | 0.4 | - | - | 20 |
| Au/ppm | 1000 | 420 | 350 | 20 |
| Ag/ppm | 2000 | 1240 | - | 200 |
| Pd/ppm | 50 | 10 | - | - |
| Ge/ppm | - | - | - | 20 |
| As/ppm | - | - | - | 10 |
| Ti/ppm | - | - | - | 200 |
| In/ppm | - | - | - | 20 |
| Ta/ppm | - | - | - | 200 |
| Co/ppm | - | - | - | 200 |
| Se/ppm | - | - | - | 20 |
| Ga/ppm | - | - | - | 10 |
| Ceramics (Max. wt. 30%) | Shuey and Taylor [16] | Kim et al. [17] | Iji and Yokoyama [18] | Ewasteguide.info [19] |
| SiO2 | 15 | 41.86 | 30 | 24.9 |
| Al2O3 | 6 | 6.97 | ||
| Alkali and alkaline earth oxides | 6 | CaO 9.95 | ||
| Titanates and mica, etc. | 3 | - | ||
| Plastics (Max. wt. 30%) | Shuey and Taylor [16] | Kim et al. [17] | Iji and Yokoyama [18] | Ewasteguide.info [19] |
| Polyethylene | 9.9 | - | Total of all plastics 16 wt% | Total of all plastics 23 wt% |
| Polypropylene | 4.8 | - | ||
| Polyesters | 4.8 | - | ||
| Epoxies | 4.8 | - | ||
| Polyvinyle chloride | 2.4 | - | ||
| Polytetraflouroethane | 2.4 | - | ||
| Nylon | 0.9 | - | ||
| PMs: | Au, Ag; |
| PGMs: | Pd, Pt, Rh, Ir and Ru; |
| BMs: | Cu, Al, Ni, Sn, Zn and Fe; |
| MCs (Hazardous): | Hg, Be, In, Pb, Cd, As and Sb; |
| SEs: | Te, Ga, Se, Ta and Ge. |
1.2. General Driving Force for E-Waste Processing
1.2.1. Environmental Concerns
| No. | Materials and Components | Description |
|---|---|---|
| 1 | Batteries | Heavy metals such as lead, mercury and cadmium are present in batteries |
| 2 | Cathode ray tubes (CTRs) | Lead in the cone glass and fluorescent coating cover the inside of panel glass |
| 3 | Mercury containing components such as switches | Mercury is used in thermostats, sensors, relays and switches (e.g., on PCBs and in measuring equipment and discharge lamps). It is also used in medical equipment, data transmission, telecommunication, and mobile phones |
| 4 | Asbestos waste | Asbestos waste has to be treated selectively |
| 5 | Toner cartridges, liquid and pasty, as well as color toner | Toner and toner cartridges have to be removed from any separately collected WEEE |
| 6 | PCBs | In PCBs, cadmium occurs in certain components, such as SMD chip resistors, infrared detectors and semiconductors |
| 7 | Polychlorinated biphenyl (PCB) containing capacitors | PCB-containing capacitors have to be removed for safe destruction |
| 8 | Liquid crystal displays (LCDs) | LCDs of a surface greater than 100 cm2 have to be removed from WEEE |
| 9 | Plastics containing halogenated flame retardants | During incineration/combustion of the plastics, halogenated flame retardants can produce toxic components |
| 10 | Equipment containing chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs) or hydrofluorocarbons (HFCs) | CFCs present in foam and refrigerating circuit must be properly extracted and destroyed. HCFCs or CFCs present in foam and refrigerating circuit must be properly extracted and destroyed or recycled |
| 11 | Gas discharge lamps | Mercury has to be removed |
1.2.2. Energy and Resource Conservation
| No. | Materials | Energy savings (%) |
|---|---|---|
| 1 | Aluminum | 95 |
| 2 | Copper | 85 |
| 3 | Iron and steel | 74 |
| 4 | Lead | 65 |
| 5 | Zinc | 60 |
| 6 | Paper | 64 |
| 7 | Plastics | >80 |
1.2.3. Economic Value of Selected PMs
| Weights% | Fe (wt%) | Al (wt%) | Cu (wt%) | Plastics (wt%) | Ag (ppm) | Au (ppm) | Pd (ppm) |
|---|---|---|---|---|---|---|---|
| TV-board | 28% | 10% | 10% | 28% | 280 | 20 | 10 |
| PCBs | 7% | 5% | 20% | 23% | 1000 | 250 | 110 |
| Mobile phone | 5% | 1% | 13% | 56% | 1380 | 350 | 210 |
| Portable audio | 23% | 1% | 21% | 47% | 150 | 10 | 4 |
| DVD-player | 62% | 2% | 5% | 24% | 115 | 15 | 4 |
| Calculator | 4% | 5% | 3% | 61% | 260 | 50 | 5 |
| Value-share | Fe | Al | Cu | Sum PMs | Ag | Au | Pd |
| TV-board | 4% | 11% | 42% | 43% | 8% | 27% | 8% |
| PCBs | 0% | 1% | 14% | 85% | 5% | 65% | 15% |
| Mobile phone | 0% | 0% | 7% | 93% | 5% | 67% | 21% |
| Portable audio | 3% | 1% | 77% | 19% | 4% | 13% | 2% |
| DVD-player | 13% | 4% | 36% | 47% | 5% | 37% | 5% |
| Calculator | 0% | 5% | 11% | 84% | 7% | 73% | 4% |
2. E-Waste Processing

2.1. Metallurgical Processes for the Extraction of Metals from E-Waste
2.1.1. Hydrometallurgical Processes

| Investigators | Leaching agent | Process conditions | Recovered metals |
|---|---|---|---|
| Park and Fray [41] | Aqua regia | Ratio of metals to leachant = 1:20 g/mL | Au, Ag and Pd |
| Sheng and Estell [49] | HNO3 (1st stage), epoxy resin (2nd stage), and aqua regia (3rd stage) | Extraction was carried out in the three stages (self agitation) | Au |
| Quinet et al. [50] | H2SO4, chloride, thiourea and cyanide leaching | Leaching & metals recovery by cementation, precipitation, ion exchange and carbon adsorption | Au, Ag, Pd and Cu |
| Chielewski et al. [51] | HNO3 and aqua regia | Roasting of e-waste in the presence of carbon; leaching with HNO3 and aqua regia; and solvent extraction with diethyle malonate | Au |
| Zhou et al. [52] | HCl, H2SO4 and NaClO3 | Combustion of e-waste at 400–500 °C followed by leaching | Ag, Au and Pd |
| Kogan [53] | HCl, MgCl2, H2SO4 and H2O2 | Dissolution of e-waste in different solvents and leaching conditions; and recovery of metals in stages | Al, Sn, Pb and Zn (1st stage), Cu and Ni (2nd stage), Au, Ag, Pd and Pt (last stage) |
| Veit et al. [11] | Aqua regia and H2SO4 | Mechanical processing and then dissolution of e-waste in different solvents | Cu |
| Mecucci and Scott [54] | HNO3 | Electrochemical deposition of Cu at cathode from solution | Pb and Cu |
Limitations of Hydrometallurgy Route
- Overall, hydrometallurgical routes are slow and time consuming and impact recycling economy. There are concerns regarding the economy of hydrometallurgical routes compared to pyrometallurgical processes for the extraction of PMs from e-waste.
- Mechanical processing of e-waste takes longer to reduce size for efficient dissolution. It is reported that 20% PM is lost by mechanical force during the liberation process that contributes to a significant loss in the overall revenue.
- Cyanide is a dangerous leachant and should therefore be used with high safety standards. It can cause contamination of rivers and seawater, especially near gold mines, which poses serious health risks to the inhabitants.
- Halide leaching is difficult to implement due to strong corrosive acids and oxidizing conditions. Specialized equipment made of stainless steel and rubbers is required for leaching of gold using halide agents from e-waste.
- The use of thiourea leachants is limited in gold extraction due to its high cost and consumption. Moreover, further developments are required to improve the current technology of thiourea-based gold leaching.
- The consumption of thiosulfate is comparatively higher and the overall process is slower, which limits its application for gold extraction from ores as well as from e-waste.
- There are risks of PM loss during dissolution and subsequent steps, therefore the overall recovery of metals will be affected.
2.1.2. Pyrometallurgical Processes
2.1.2.1. Lead Smelting Route
2.1.2.2. Copper Smelting Route
2.1.2.3. Limitations of Pyrometallurgical Processes
- Recovery of plastics is not possible because plastics replace coke as a source of energy;
- Iron and aluminum recovery is not easy as they end up in the slag phase as oxides;
- Hazardous emissions such as dioxins are generated during smelting of feed materials containing halogenated flame retardants. Therefore special installations are required to minimize environmental pollution;
- A large investment is required for installing integrated e-waste recycling plants that maximize the recovery of valuable metals and also protect the environment by controlling hazardous gas emissions;
- Instant burning of fine dust of organic materials (e.g., non-metallic fractions of e-waste) can occur before reaching the metal bath. In such cases, agglomeration may be required to effectively harness the energy content and also to minimize the health risk posed by fine dust particles;
- Ceramic components in feed material can increase the volume of slag generated in the blast furnaces, which thereby increases the risk of losing PMs from BMs;
- Partial recovery and purity of PMs are achieved by pyrometallurgical routes. Therefore, subsequent hydrometallurgical and electrochemical techniques are necessary to extract pure metals from BMs;
- Handling the process of smelting and refining is challenging due to complex feed materials. The expertise in process handling and the thermodynamics of possible reactions will be difficult.
3. Industrial Processes for the Recovery of Metals from E-Waste
3.1. Umicore Integrated Metals Smelters and Refinery



3.2. Metals Recovery from E-Waste at Rönnskär Smelters

3.3. Noranda Process

- It maximizes the overall segregation of PMs in the copper fraction, and, therefore, the final recovery will be higher;
- It partially replaces coke with plastics as a source of energy during the smelting process;
- It provides a source for recycling of e-waste;
- Moreover, it is efficient in terms of resource management by closing the loop of metals.
| Umicore’s process [20,60,61] | Au, Ag, Pd, Pt, Se, Ir, Ru, Rh, Cu, Ni, Pb, In, Bi, Sn, As, Sb | Isasmelt smelting, copper leaching & electrowinning and PMs refinery |
| Outotec TSL [66] | Zn, Cu, Au, Ag, In, Pb, Cd, Ge | Ausmelt TSL furnace (trials in Melbourne, Australia), smelting of e-waste in copper/lead/zinc processes |
| Rönnskär smelters [63,67] | Cu, Ag, Au, Pd, Ni, Se, Zn, Pb | Smelting in Kaldo reactor, upgrading in copper and followed by refining, high PMs recovery |
| Noranda process [64] | Cu, Au, Ag, Pt, Pd, Se, Te, Ni | Smelting of e-waste and Cu concentrate. Upgrading in converter and anode furnaces. Electrorefining for metal recovery |
| Rönnskär smelters tests [63,68] | Cu and PMs | PC scrap feeding to Zn fuming process, Plastics is used as reducing agent, PMs are segregated in Cu and are recovered at later stage |
| Umicore’s trials [69] | Au, Ag, Pd, Pt, Se, Ir, Ru, Rh, Cu, Ni, Pb, In, Bi, Sn, As, Sb | Plastics from e-waste is tested at energy and reducing agent during smelting |
| Dowa mining Kosaka Japan [70] | Cu, Au, Ag | E-waste TSL smelting in secondary copper process |
| LS-Nikko’s recycling facility, Korea [71] | Au, Ag & PGMs metals | Recycling in TSL smelting followed by electrolytic refining |
| Day’s patent [72] | PMs, Pt and Pd | Smelting in plasma arc furnace at 1400 °C. PMs collected in BM. Ceramic residue went in the slag phase. Ag and Cu used to collect metals during process |
| Aleksandrovich patent [73] | PGMs and gold | Scrap combustion in a BM using carbon as reducing agent, Solidification and separation of solidified product are carried out by formed phase boundaries |
| Aurubis recycling Germany [74] | Cu, Pb, Zn, Sn and PMs | Smelting of Cu and e-waste in TSL reactor, black copper processing and finally electrorefining |
4. Design for Resource (DfR) Efficiency
5. E-Waste Recycling in Australia

6. Challenges for the Recycling of E-Waste in Australia
6.1. Lack of Facilities for E-Waste Collection
6.2. Transportation (Cost and Distance)
6.3. Lack of Facilities for Separating Metals from Complex E-Waste Materials
6.4. Technical Barriers—Knowledge of Process Thermodynamics
6.5. Lack of Integrated Smelting and Refining Facility
6.6. Economic Barriers
6.7. Direct Recycled Metal Manufacturing
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef]
- European Parliament. Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on waste electrical and electronic equipment (WEEE). Off. J. Eur. Union 2003, L37, 24–38. [Google Scholar]
- European Parliament. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on waste electrical and electronic equipment (WEEE). Off. J. Eur. Union 2012, L197, 38–71. [Google Scholar]
- Puckett, J.; Byster, L.; Westervelt, S.; Gutierrez, R.; Davis, S.; Hussain, A.; Dutta, M. Exporting Harm—The High-Tech Trashing of Asia; The Basel Action Network (BAN) Silicon Valley Toxics Coalition (SVTC): Seattle, WA, USA, 2002. [Google Scholar]
- Association of Plastics Manufacturers in Europe (APME). Plastics—A Material of Choice for the Electrical and Electronic Industry-Plastics Consumption and Recovery in Western Europe 1995; APME: Brussels, Belgium, 2004; p. 1. [Google Scholar]
- Ogunniyi, I.O.; Vermaak, M.K.G.; Groot, D.R. Chemical composition and liberation characterization of printed circuit board comminution fines for beneficiation investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef]
- Luda, M.P. Recycling of printed circuit boards. Integr. Waste Manag. 2010, 2, 285–299. [Google Scholar]
- Moltó, J.; Font, R.; Gálvez, A.; Conesa, J.A. Pyrolysis and combustion of electronic wastes. J. Anal. Appl. Pyrolysis 2009, 84, 68–78. [Google Scholar] [CrossRef]
- Wienold, J.; Recknagel, S.; Scharf, H.; Hoppe, M.; Michaelis, M. Elemental analysis of printed circuit boards considering the ROHS regulations. Waste Manag. 2011, 31, 530–535. [Google Scholar] [CrossRef]
- Jie, G.; Ying-Shun, L.; Mai-Xi, L. Product characterization of waste printed circuit board by pyrolysis. J. Anal. Appl. Pyrolysis 2008, 83, 185–189. [Google Scholar] [CrossRef]
- Veit, H.M.; Bernardes, A.M.; Ferreira, J.Z.; Tenório, J.A.; de Fraga Malfatti, C. Recovery of copper from printed circuit boards scraps by mechanical processing and electrometallurgy. J. Hazard. Mater. 2006, 137, 1704–1709. [Google Scholar]
- Li, J.; Xu, Z.; Zhou, Y. Application of corona discharge and electrostatic force to separate metals and nonmetals from crushed particles of waste printed circuit boards. J. Electrost. 2007, 65, 233–238. [Google Scholar] [CrossRef]
- Lu, H.; Li, J.; Guo, J.; Xu, Z. Movement behavior in electrostatic separation: Recycling of metal materials from waste printed circuit board. J. Mater. Process. Technol. 2008, 197, 101–108. [Google Scholar] [CrossRef]
- Yamane, L.H.; de Moraes, V.T.; Espinosa, D.C.; Tenório, J.A. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Z. Recycling of non-metallic fractions from waste printed circuit boards: A review. J. Hazard. Mater. 2009, 168, 567–590. [Google Scholar] [CrossRef]
- Shuey, S.A.; Taylor, P. A Review of Pyrometallurgical Treatment of Electronic Scrap. In Proceedings of the SME Annual Meeting, Denver, CO, USA, 23–25 February 2004.
- Kim, B.S.; Lee, J.C.; Seo, S.P.; Park, Y.K.; Sohn, H.Y. A process for extracting precious metals from spent printed circuit boards and automobile catalysts. JOM 2004, 56, 55–58. [Google Scholar]
- Iji, M.; Yokoyama, S. Recycling of printed wiring boards with mounted electronic components. Circuit World 1997, 23, 10–15. [Google Scholar] [CrossRef]
- The Composition of Valuable Substances in E-Waste. Available online: http://ewasteguide.info (accessed on 19 November 2013).
- Hagelüken, C. Improving Metal Returns and Eco-Efficiency in Electronics Recycling—A Holistic Approach for Interface Optimisation between Pre-Processing and Integrated Metals Smelting and Refining. In Proceedings of the IEEE International Symposium on Electronics and the Environment, Scottsdale, AZ, USA, 8–11 May 2006.
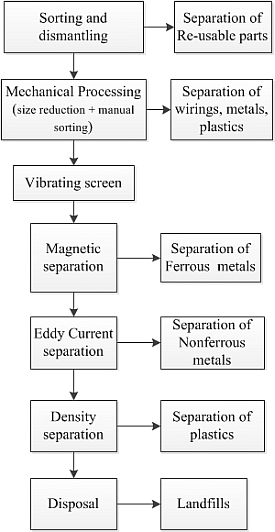
- Cui, J.; Forssberg, E. Mechanical recycling of waste electric and electronic equipment: A review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef]
- Office of Solid Waste. Electronic Waste Management in the United States—Approach 1; U.S Environmental Protection Agency: Washington, DC, USA, 2008; p. 56.
- Widmer, R.; Oswald-Krapf, H.; Sinha-Khetriwal, D.; Schnellmann, M.; Böni, H. Global perspectives on e-waste. Environ. Impact Assess. Rev. 2005, 25, 436–458. [Google Scholar] [CrossRef]
- Kang, H.-Y.; Schoenung, J.M. Electronic waste recycling: A review of U.S. infrastructure and technology options. Resour. Conserv. Recycl. 2005, 45, 368–400. [Google Scholar] [CrossRef]
- Kahhat, R.; Kim, J.; Xu, M.; Allenby, B.; Williams, E.; Zhang, P. Exploring e-waste management systems in the United States. Resour. Conserv. Recycl. 2008, 52, 955–964. [Google Scholar] [CrossRef]
- Lee, J.-C.; Song, H.T.; Yoo, J.-M. Present status of the recycling of waste electrical and electronic equipment in Korea. Resour. Conserv. Recycl. 2007, 50, 380–397. [Google Scholar] [CrossRef]
- Huang, K.; Guo, J.; Xu, Z. Recycling of waste printed circuit boards: A review of current technologies and treatment status in China. J. Hazard. Mater. 2009, 164, 399–408. [Google Scholar] [CrossRef]
- Hischier, R.; Wäger, P.; Gauglhofer, J. Does WEEE recycling make sense from an environmental perspective?: The environmental impacts of the Swiss take-back and recycling systems for waste electrical and electronic equipment (WEEE). Environ. Impact Assess. Rev. 2005, 25, 525–539. [Google Scholar] [CrossRef]
- Dalrymple, I.; Wright, N.; Kellner, R.; Bains, N.; Geraghty, K.; Goosey, M.; Lightfoot, L. An integrated approach to electronic waste (WEEE) recycling. Circuit World 2007, 33, 52–58. [Google Scholar] [CrossRef]
- Institute of Scrap Recycling Industries (ISRI). Scrap Recycling: Where Tomorrow Begins; ISRI: Washington, DC, USA, 2003; pp. 16–24. [Google Scholar]
- Rankin, J. Minerals, Metals and Sustainability—Meeting Future Materials Needs; Commonwealth Scientific and Industrial Research Organization (CSIRO): Melbourne, Australia, 2011. [Google Scholar]
- Meskers, C.E.M.; Hagelüken, C.; Salhofer, S.; Spitzbart, M. Impact of Pre-Processing Routes on Precious Metal Recovery from PCs. In Proceedings of the European Metallurgical Conference (EMC), Innsbruck, Austria, 28 June–1 July 2009.
- Guo, J.; Cao, B.; Guo, J.; Xu, Z. A plate produced by nonmetallic materials of pulverized waste printed circuit boards. Environ. Sci. Technol. 2008, 42, 5267–5271. [Google Scholar]
- Yin, J.; Li, G.; He, W.Z. Preparation of a Composite plate Using Nonmetallic Materials Powder from the Waste Printed Circuit Boards. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010.
- Sohaili, J.; Muniyandi, S.K.; Mohamad, S.S. A review on printed circuit boards waste recycling technologies and reuse of recovered nonmetallic materials. Int. J. Sci. Eng. Res. 2012, 3, 138–144. [Google Scholar]
- Anindya, A. Minor Elements Distribution during the Smelting of WEEE with Copper Scrap. Ph.D Thesis, RMIT University, Melbourne, Australia, 2012. [Google Scholar]
- Chehade, Y.; Siddique, A.; Alayan, H.; Sadasivam, N.; Nusri, S.; Ibrahim, T. Recovery of Gold, Silver, Palladium, and Copper from Waste Printed Circuit Boards. In Proceedings of the International Conference on Chemical, Civil and Environment Engineering (ICCEE), Dubai, United Arab Emirates, 24–25 March 2012.
- Dhawan, N.; Kumar, M.; Kumar, V.; Wadhwa, M. Recovery of Metals from Electronic Scrap by Hydrometallurgical Route. In Proceedings of the Global Symposium on Recycling Waste Treatment and Clean Technology (REWAS), Cancun, Mexico, 12–15 October 2008; pp. 693–698.
- Dhawan, N.; Kumar, V.; Kumar, M. Recovery of Metals from Electronic Scrap by Hydrometallurgical Route. In Extraction and Processing Division (EPD) Congress; The Minerals, Metals and Materials Society: Warrendale, PA, USA, 2009; pp. 1107–1109. [Google Scholar]
- Delfini, M.; Ferrini, M.; Manni, A.; Massacci, P.; Piga, L. Antonio Scoppettuolo Optimization of precious metal recovery from waste electrical and electronic equipment boards. J. Environ. Prot. 2011, 2, 675–682. [Google Scholar]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 164, 1152–1158. [Google Scholar] [CrossRef]
- Sadegh Safarzadeh, M.; Bafghi, M.S.; Moradkhani, D.; Ojaghi Ilkhchi, M. A review on hydrometallurgical extraction and recovery of cadmium from various resources. Miner. Eng. 2007, 20, 211–220. [Google Scholar]
- Ritcey, G.M. Solvent extraction in hydrometallurgy: Present and future. Tsinghua Sci. Technol. 2006, 11, 137–152. [Google Scholar] [CrossRef]
- Yang, B. Ion exchange in organic extractant system. Ion Exch. Adsorpt. 1994, 10, 168–179. [Google Scholar]
- Shamsuddin, M. Metal recovery from scrap and waste. J. Metals 1986, 38, 24–31. [Google Scholar]
- Tavlarides, L.L.; Bae, J.H.; Lee, C.K. Solvent extraction, membranes, and ion exchange in hydrometallurgical dilute metals separation. Sep. Sci. Technol. 1985, 22, 581–617. [Google Scholar]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Paretsky, V.M.; Antipov, N.I.; Tarasov, A.V. Hydrometallurgical Method for Treating Special Alloys, Jewelry, Electronic and Electrotechnical Scrap. In Proceedings of the Minerals, Metals & Materials Society (TMS) Annual Meeting, Charlotte, NC, USA, 14–18 March 2004; pp. 713–721.
- Sheng, P.P.; Etsell, T.H. Recovery of gold from computer circuit board scrap using aqua regia. Waste Manag. Res. 2007, 25, 380–383. [Google Scholar] [CrossRef]
- Quinet, P.; Proost, J.; van Lierde, A. Recovery of precious metals from electronic scrap by hydrometallurgical processing routes. Miner. Metall. Process. 2005, 22, 17–22. [Google Scholar]
- Chmielewski, A.G.; Urbański, T.S.; Migdał, W. Separation technologies for metals recovery from industrial wastes. Hydrometallurgy 1997, 45, 333–344. [Google Scholar] [CrossRef]
- Zhou, P.; Zheng, Z.; Tie, J. Technological Process for Extracting Gold, Silver and Palladium from Electronic Industry Waste. Chin. Patent 1603432, 6 April 2005. [Google Scholar]
- Kogan, V. Process for the Recovery of Precious Metals from Electronic Scrap by Hydrometallurgical Technique. Int. Patent WO/2006/013568, 9 February 2006. [Google Scholar]
- Mecucci, A.; Scott, K. Leaching and electrochemical recovery of copper, lead and tin from scrap printed circuit boards. J. Chem. Technol. Biotechnol. 2002, 77, 449–457. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of gold extraction from ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Antrekowitsch, H.; Potesser, M.; Spruzina, W.; Prior, F. Metallurgical Recycling of Electronic Scrap. In Proceedings of the EPD Congress, San Antonio, TX, USA, 12–16 March 2006; pp. 12–16.
- US Environmental Protection Agency (EPA). Lead Smelting Process. Available online: http://www.epa.gov/ttn/chief/ap42/ch12/final/c12s06.pdf (accessed on 2 December 2013).
- Anindya, A.; Swinbourne, D.R.; Reuter, M.A.; Matusewicz, R.W. Distribution of elements between copper and FeOx-CaO-SiO2 slags during pyrometallurgical processing of WEEE. Miner. Process. Extr. Metall. 2013, 122, 165–173. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling of electronic scrap at Umicore’s integrated metals smelter and refinery. Proc. EMC 2005, 59, 152–161. [Google Scholar]
- Hagelüken, C. Recycling of electronic scrap at Umicore precious metals refining. Acta Metall. Slov. 2006, 12, 111–120. [Google Scholar]
- Reuter, M.A. Metal Recycling—Opportunities, Limits and Infrastructure; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2013. [Google Scholar]
- Mark, F.E.; Lehner, T. Plastics Recovery from Waste Electrical and Electronic Equipment in Non-Ferrous Metal Processes; Association of Plastics Manufacturers in Europe: Brussels, Belgium, 2000. [Google Scholar]
- Veldbuizen, H.; Sippel, B. Mining discarded electronics. Ind. Environ. 1994, 17, 7–11. [Google Scholar]
- Huisman, J.; Stevels, L.N. Eco-efficiency of take-back and recycling, a comprehensive approach. IEEE Trans. Electron. Packag. Manuf. 2006, 29, 83–90. [Google Scholar] [CrossRef]
- Hoang, J.; Reuter, M.A.; Matusewicz, R.; Hughes, S.; Piret, N. Top submerged lance direct zinc smelting. Miner. Eng. 2009, 22, 742–751. [Google Scholar] [CrossRef]
- Lehner, T.; Vikdahl, A. Integrated Recycling of Non-Ferrous Metals at Boliden Ltd. Ronnskar Smelter. In Proceedings of the IEEE International Symposium on Electronics and the Environment (ISEE), Oak Brook, IL, USA, 4–6 May 1998; pp. 42–47.
- Lehner, T.; Wiklund, J. Sustainable Production: The business of Non-Ferrous Smelting in Sweden. In Proceedings of the International Congress on Mineral Processing and Extractive Metallurgy, Melbourne, Australia, 11–13 September 2000.
- Meskers, C.E.M.; Hagelüken, C.; van Damme, G. Green Recycling of EEE: Special and Precious Metal Recovery from EEE. In Extraction and Processing Division (EPD) Congress; The Minerals, Metals and Materials Society: Warrendale, PA, USA, 2009. [Google Scholar]
- Maeda, Y.; Inoue, H.; Kawamura, S.; Ohike, H. Metal Recycling at Kosaka Smelter. In Proceedings of the 4th International Symposium on Recycling of Metals and Engineered Materials, Pittsburgh, PA, USA, 22–25 October 2000.
- Nikko, L. Pride in Value Smelter—Smelt Passion, Refine Future; LS-Nikko Copper Inc.: Seoul, Korea, 2013. [Google Scholar]
- Day, J. Recovery of Platinum Group Metals, Gold and Silver from Scrap. U.S. Patents 4,427,442, 24 January 1984. [Google Scholar]
- Aleksandrovich, S.; Nicolaevich, E.; Ivanovich, E. Method of Processing of Products Based on Ahalcogenides of Base Metals Containing Metals of Platinum Group and Gold. Russ. Patent 2112064, 11 February 1998. [Google Scholar]
- Recycling Technology—Aurubis’ Multi-Metal Recycling. Available online: http://www.aurubis.com/en/our-business/raw-materials/recycling/technology/ (accessed on 24 November 2013).
- Tanskanen, P. Management and recycling of electronic waste. Acta Mater. 2013, 61, 1001–1011. [Google Scholar] [CrossRef]
- Westcott, M. E-Waste; Queensland Parliamentary: Queensland, Australia, 2012. [Google Scholar]
- Pink, R. Australia’s Environment: Issues and Trends; Australian Bureau of Statistics: Canberra, Australia, 2010. [Google Scholar]
- Baker, E.; Bournay, E.; Harayama, A.; Rekacewicz, P. Vital Waste Graphics; GRID-Arendal: Arendal, Norway, 2005. [Google Scholar]
- Local Government Association of South Australia. Regulatory Requirements for the Collection, Handling, Transporting and Recycling of E-Waste.—Circular 23.4. Available online: https://www.lga.sa.gov.au/page.aspx?c=28933 (accessed on 18 November 2013).
- Australian Bureau of Statistics. Electronic and Electrical Waste, 2013. Available online: http://www.abs.gov.au/ausstats/[email protected]/Products/4602.0.55.005~2013~Main+Features~Electronic+and+Electrical+Waste?OpenDocument (accessed on 18 November 2013).
- Sims Recycling Solutions. Sims Metal Management. Available online: http://us.simsrecycling.com/About-Us/Sims-Metal-Management (accessed on 22 November 2013).
- Demunck, T. E-waste Recycling in Australia. In Proceedings of the Personal Communication with Sims Metal Management, Melbourne, Australia, 7 November 2013.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khaliq, A.; Rhamdhani, M.A.; Brooks, G.; Masood, S. Metal Extraction Processes for Electronic Waste and Existing Industrial Routes: A Review and Australian Perspective. Resources 2014, 3, 152-179. https://0-doi-org.brum.beds.ac.uk/10.3390/resources3010152
Khaliq A, Rhamdhani MA, Brooks G, Masood S. Metal Extraction Processes for Electronic Waste and Existing Industrial Routes: A Review and Australian Perspective. Resources. 2014; 3(1):152-179. https://0-doi-org.brum.beds.ac.uk/10.3390/resources3010152
Chicago/Turabian StyleKhaliq, Abdul, Muhammad Akbar Rhamdhani, Geoffrey Brooks, and Syed Masood. 2014. "Metal Extraction Processes for Electronic Waste and Existing Industrial Routes: A Review and Australian Perspective" Resources 3, no. 1: 152-179. https://0-doi-org.brum.beds.ac.uk/10.3390/resources3010152