Multifunctional Biological Properties and Topical Film Forming Spray Base on Auricularia polytricha as a Natural Polysaccharide Containing Brown Agaricus bisporus Extract for Skin Hydration
Abstract
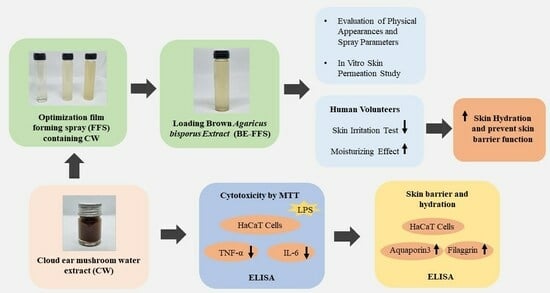
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cloud Ear Mushroom Extraction
2.3. Determination of Total Polysaccharides Content
2.4. Cell Culture
2.4.1. Determination of Cytotoxicity
2.4.2. Quantification of IL-6 and TNF-α Secretions by ELISA
2.4.3. Expression of FLG and AQP3 by ELISA
2.5. Optimization of Film Forming Spray Base
2.6. Preparation of Film Forming Spray Containing Brown A. bisporus Extract
2.7. Evaluation of the BE-FFS
2.7.1. Film Forming Characteristics
2.7.2. Drying Time
2.7.3. Occlusive Factor
2.7.4. Spray Pattern and Spray Angle
2.8. In Vitro Skin Permeation Study of BE-FFS
2.9. Human Skin Irritation Test
2.10. Moisturizing Effect of BE-FFS in Human Volunteers
2.11. Statistical Analysis
3. Results and Discussion
3.1. Cloud Ear Mushroom Extraction
3.2. Determination of Total Polysaccharides Content
3.3. Cell Culture
3.3.1. Cytotoxicity Test by MTT Assay
3.3.2. Preventive Effect of Cloud Ear Mushroom Extract against Inflammation in LPS-Induced HaCaT Cells
3.3.3. Expression of AQP3 and FLG
3.4. Optimization of Film Forming Spray Base
3.5. Evaluation of Physical Appearances and Spray Parameters of BE-FFS
3.6. In Vitro Skin Permeation Study of BE-FFS
3.7. Skin Irritation Test in Human Volunteers
3.8. Moisturizing Effect of BE-FFS in Human Volunteers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawlings, A.V.; Scott, I.R.; Harding, C.R.; Bowser, P.A. Stratum corneum moisturization at the molecular level. J. Investig. Dermatol. 1994, 103, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J. Understanding the role of natural moisturizing factor in skin hydration. Pract. Dermatol. 2012, 9, 36–40. [Google Scholar]
- Rawlings, A.V.; Matts, P.J. Stratum corneum moisturization at the molecular level: An update in relation to the dry skin cycle. J. Investig. Dermatol. 2005, 124, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hara, M.; Sougrat, R.; Verbavatz, J.-M.; Verkman, A. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 2002, 277, 17147–17153. [Google Scholar] [CrossRef]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Umar, A.K.; Butarbutar, M.; Sriwidodo, S.; Wathoni, N. Film forming sprays for topical drug delivery. Drug Des. Dev. Ther. 2020, 14, 2909–2925. [Google Scholar] [CrossRef]
- Mohd, T.A.T.; Manaf, S.F.A.; Abd Naim, M.; Shayuti, M.S.M.; Jaafar, M.Z. Properties of Biodegradable Polymer from Terrestrial Mushroom for Potential Enhanced Oil Recovery. Indones. J. Chem. 2020, 20, 1382–1391. [Google Scholar] [CrossRef]
- Sillapachaiyaporn, C.; Chuchawankul, S.; Nilkhet, S.; Moungkote, N.; Sarachana, T.; Ung, A.T.; Baek, S.J.; Tencomnao, T. Ergosterol isolated from cloud ear mushroom (Auricularia polytricha) attenuates bisphenol A-induced BV2 microglial cell inflammation. Food Res. Int. 2022, 157, 111433. [Google Scholar] [CrossRef]
- Ngwuluka, N.C.; Ochekpe, N.A.; Aruoma, O.I. Functions of bioactive and intelligent natural polymers in the optimization of drug delivery. Ind. Appl. Intell. Polym. Coat. 2016, 6, 165–184. [Google Scholar]
- Packialakshmi, B.; Sudha, G.; Charumathy, M. Studies on phytochemical compounds and antioxidant potential of Auricularia auricula-judae. Int. J. Pharm. Sci. Res. 2017, 8, 3508–3515. [Google Scholar]
- Nitthikan, N.; Leelapornpisid, P.; Naksuriya, O.; Intasai, N.; Kiattisin, K. Potential and alternative bioactive compounds from brown Agaricus bisporus mushroom extracts for xerosis treatment. Sci. Pharm. 2022, 90, 59. [Google Scholar] [CrossRef]
- Hassan, M.J.; Karim, M.M.; Islam, M.A.; Pramanik, M.H.R.; Hossain, M.A. Changes in root porosity and water-soluble carbohydrates in rice (Oryza sativa L.) under submergence stress. J. Bangladesh Agric. Univ. 2019, 17, 539–544. [Google Scholar] [CrossRef]
- Paradkar, M.; Thakkar, V.; Soni, T.; Gandhi, T.; Gohel, M. Formulation, and evaluation of clotrimazole transdermal spray. Drug Dev. Ind. Pharm. 2015, 41, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.; Bajaj, A.; Londhe, V.; Babul, N.; Kao, D. Fabrication of topical metered dose film forming sprays for pain management. Eur. J. Pharm. Sci. 2017, 100, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhuang, C.; Gu, W.; Zhao, Y. Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique. Carbohydr. Polym. 2019, 212, 197–205. [Google Scholar] [CrossRef]
- Nitthikan, N.; Leelapornpisid, P.; Natakankitkul, S.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Kiattisin, K. Improvement of stability and transdermal delivery of bioactive compounds in green robusta coffee beans extract loaded nanostructured lipid carriers. J. Nanotechnol. 2018. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Clarys, P.; Clijsen, R.; Taeymans, J.; Barel, A.O. Hydration measurements of the stratum corneum comparison between the capacitance method (digital version of the Corneometer CM 825®) and the impedance method (S kicon-200 EX®). Ski. Res. Technol. 2012, 18, 316–323. [Google Scholar] [CrossRef]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.; Bruze, M.; Cannavó, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A. European Society of Contact Dermatitis guideline for diagnostic patch testing–recommendations on best practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef]
- Farage, M.A.; Maibach, H.I.; Andersen, K.E.; Lachapelle, J.M.; Kern, P.; Ryan, C.; Ely, J.; Kanti, A. Historical perspective on the use of visual grading scales in evaluating skin irritation and sensitization. Contact Dermat. 2011, 65, 65–75. [Google Scholar] [CrossRef]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroalga [Rhizoclonium hieroglyphicum (C. Agardh) Kützing] for skin care cosmetic. Chiang Mai J. Sci. 2014, 41, 1195–1207. [Google Scholar]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef] [PubMed]
- Merdivan, S.; Bettin, P.; Preisitsch, M.; Lindequist, U. Quality control of medicinal mushrooms: Comparison of different methods for the quantification of polysaccharides/β-glucans. Planta Medica 2014, 80, P1N22. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G. Extraction and derivatisation of active polysaccharides. J. Enzym. Inhib. Med. Chem. 2019, 34, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, R.M.; Sloan, D.K.; Heck, J.M.; Murray, A.R.; O’Dell, S.J. Interleukin 6 indirectly induces keratinocyte migration. J. Investig. Dermatol. 2004, 122, 764–772. [Google Scholar] [CrossRef]
- Lu, B.; Elias, P.M.; Feingold, K.R. The Role of the Primary Cytokines, TNF, IL-1, and IL-6, in Permeability Barrier Homeostasis. Ski. Barrier 2005, 1, 325–338. [Google Scholar]
- Zhu, X.; Jiang, L.; Zhong, Q.; Kong, X.; Zhang, R.; Zhu, L.; Liu, Q.; Wu, W.; Tan, Y.; Wang, J. Abnormal expression of interleukin-6 is associated with epidermal alternations in localized scleroderma. Clin. Rheumatol. 2022, 41, 2179–2187. [Google Scholar] [CrossRef]
- Hiramoto, K.; Goto, K.; Tanaka, S.; Horikawa, T.; Ooi, K. Skin liver and kidney interactions contribute to skin dryness in aging KK-Ay/Tajcl mice. Biomedicines 2022, 10, 2648. [Google Scholar] [CrossRef]
- Shen, X.; Guo, M.; Yu, H.; Liu, D.; Lu, Z.; Lu, Y. Propionibacterium acnes related anti-inflammation and skin hydration activities of madecassoside, a pentacyclic triterpene saponin from Centella asiatica. Biosci. Biotechnol. Biochem. 2019, 83, 561–568. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- Sarkar, S.; Mondal, M.; Ghosh, P.; Saha, M.; Chatterjee, S. Quantification of total protein content from some traditionally used edible plant leaves: A comparative study. J. Med. Plant Stud. 2020, 8, 166–170. [Google Scholar] [CrossRef]
- Mori, N.M.; Patel, P.; Sheth, N.R.; Rathod, L.V.; Ashara, K.C. Fabrication and characterization of film-forming voriconazole transdermal spray for the treatment of fungal infection. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 41–51. [Google Scholar] [CrossRef]
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue bioactive components pharmacology and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiang, H.; Zhang, Q.; Miao, X. Polysaccharide-Based Transdermal Drug Delivery. Pharmaceuticals 2022, 15, 602. [Google Scholar] [CrossRef] [PubMed]
- Fodil-Bourahla, I.; Bizbiz, L.; Schoevaert, D.; Robert, A.; Robert, L. Effect of L-fucose and fucose-rich oligo-and polysaccharides (FROP-s) on skin aging penetration skin tissue production and fibrillogenesis. Biomed. Pharmacother. 2003, 57, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Katekawa, E.; Caverzan, J.; Mussi, L.; Camargo-Junior, F.B.; Sufi, B.; Padovani, G.; Nazato, L.; Nogueira, C.; Magalhães, W.V.; Di Stasi, L.C. Novel topical skin hydration agent containing Anadenanthera colubrina polysaccharide-standardized herbal preparation. J. Cosmet. Dermatol. 2020, 19, 1691–1698. [Google Scholar] [CrossRef]
- Pacheco, M.S.; Barbieri, D.; da Silva, C.F.; de Moraes, M.A. A review on orally disintegrating films (ODFs) made from natural polymers such as pullulan, maltodextrin, starch, and others. Int. J. Biol. Macromol. 2021, 178, 504–513. [Google Scholar] [CrossRef]







| RUN | Independent Variable (%) | ||
|---|---|---|---|
| A (CW) | B (SDS) | C (GLY) | |
| 1 | 0 | 0 | 3 |
| 2 | 2 | 0.5 | 0.5 |
| 3 | 3 | 0 | 0 |
| 4 | 1.5 | 1.5 | 0 |
| 5 | 1 | 1 | 1 |
| 6 | 0 | 1.5 | 1.5 |
| 7 | 0.5 | 2 | 0.5 |
| 8 | 0.5 | 0.5 | 2 |
| 9 | 1.5 | 0 | 1.5 |
| 10 | 0 | 3 | 0 |
| 11 | 0 | 3 | 0 |
| 12 | 3 | 0 | 0 |
| BE-FFS | Flux (µg/cm2/h) | Permeability Coefficient, Kp (cm/h) × 10−6 | Cumulative Amount, Q12 (μg/cm2) |
|---|---|---|---|
| ERT | 0.042 ± 0.11 | 8.34 ± 0.27 | 49.78 ± 0.62 |
| GA | 0.011 ± 0.15 | 1.30 ± 0.31 | 13.07 ± 0.12 |
| Test Substances | PDII Value | Classification of Skin Reaction |
|---|---|---|
| BE-FFS | 0.03 | No irritation |
| Positive (2% w/v SLS) | 0.50 | Slight irritation |
| Negative (DI water) | 0.03 | No irritation |
| Weeks | Skin Hydration (a.u.) | Skin Moisturizing Efficacy (%) | TEWL (g/m2/h) | TEWL Protection Efficacy (%) |
|---|---|---|---|---|
| 0 | 38.47 ± 6.01 a | 27.3 ± 9.55 | 14.20 ± 8.10 b | −17.9 ± 5.60 |
| 4 | 48.43 ± 8.03 b | 9.60 ± 2.30 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitthikan, N.; Leelapornpisid, P.; Naksuriya, O.; Intasai, N.; Kiattisin, K. Multifunctional Biological Properties and Topical Film Forming Spray Base on Auricularia polytricha as a Natural Polysaccharide Containing Brown Agaricus bisporus Extract for Skin Hydration. Cosmetics 2023, 10, 145. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics10050145
Nitthikan N, Leelapornpisid P, Naksuriya O, Intasai N, Kiattisin K. Multifunctional Biological Properties and Topical Film Forming Spray Base on Auricularia polytricha as a Natural Polysaccharide Containing Brown Agaricus bisporus Extract for Skin Hydration. Cosmetics. 2023; 10(5):145. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics10050145
Chicago/Turabian StyleNitthikan, Nichcha, Pimporn Leelapornpisid, Ornchuma Naksuriya, Nutjeera Intasai, and Kanokwan Kiattisin. 2023. "Multifunctional Biological Properties and Topical Film Forming Spray Base on Auricularia polytricha as a Natural Polysaccharide Containing Brown Agaricus bisporus Extract for Skin Hydration" Cosmetics 10, no. 5: 145. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics10050145