1. Introduction
Lip histology is well defined, with this region consisting of a tenuous stratum corneum (SC), formed by orthokeratotic cells that renew more quickly than those present in the normal SC, and the epithelium characterized as a thin tissue, slightly keratinized and with less ceramide content [
1]. Due to their prominent location in the facial region, the lips are constantly susceptible to influences from the external environment, such as solar radiation, wind, extreme temperatures and the use of cosmetics and dental treatments [
2]. Of the different skin maintenance mechanisms, the hydration state of the SC is the most commonly altered on a daily basis, and, for an intact barrier to be maintained in the epidermis, an adequate amount of water needs to be present on this surface [
3]. Due to the rapid cell renewal of the lip SC, immature corneocytes are exposed to the skin surface, allowing the water present in the lips to transpire more easily, resulting in a dry and rough region [
4]. In order to prevent dryness and roughness of the lips, maintaining or increasing SC hydration levels, cosmetic products for lip treatment are an excellent alternative [
3].
In recent years, there has been a growing demand for the development of more natural and sustainable cosmetics, mainly through the dissemination of these ideals through social media, which influence consumers’ opinion and purchase of products [
5]. In this way, research by the cosmetic industry into natural actives and inputs that are safe for human use has been highlighted [
6]. These natural actives can be obtained from different sources, such as vegetal, which includes oils, butters, waxes, essential oils, among others; animal, which includes waxes, honey [
7] and others; and microbial, which includes biopolymers and biomolecules and may provide additional benefits to the cosmetic formulation.
The global dermocosmetics market is expected to grow at a compound annual growth rate (CAGR) of 7.5% until 2030, due to investment and development of new skin and hair care solutions by the industry [
8]. Cosmeceuticals, which are cosmetic products with medicinal and drug-like benefits, have been a growing demand in the last years, especially due to research into bioactive molecules, which present biological properties such as repairing, moisturizing and anti-aging [
9] and renewable characteristics. This research has been carried out by the cosmetic industry in association with biotechnology, a science that allows the development of new inputs and products efficiently [
10] through fermentative processes or genetic engineering using microorganisms and enzymes, which can help in the evaluation of these components in the skin [
11].
Examples of biotechnological molecules used in cosmetic formulations correspond to polysaccharides and derived lipids, such as levan (LEV) and sophorolipids (SOPs) [
12]. LEV corresponds to a fructose exopolysaccharide (EPS), formed through glycosidic bonds of the β(2→6) type [
13], which can be obtained by fermentative processes from a variety of microorganisms, such as
Bacillus subtilis natto. This EPS has several bioactive properties of cosmetic interest, such as moisturizing, antioxidant and filler effects [
14]. Kim et al. [
15] evaluated the properties presented by LEV from
Zymomonas mobilis, and they verified that this molecule has moisturizing properties similar to hyaluronic acid, as well as similar proliferation of human fibroblasts and keratinocyte cells, which demonstrates that LEV can be used as a cosmeceutical agent. Choi et al. [
16] evaluated the potential of LEV as a dermal filler. They verified that levan-based hydrogel enhanced cell proliferation, showed better collagen synthesis than hyaluronic acid and also demonstrated anti-wrinkle efficacy. Pei et al. [
17], Domżał-Kędzia et al. [
18] and Bouallegue et al. [
19] reported that LEV presents great antioxidant activity due to its capacity to donate electrons to the free radicals. Da Silva et al. [
20] developed a facial cosmetic formulation containing LEV (0.75 g) and almond oil and evaluated its spreadability, antioxidant and moisture-retention capacity. They verified that the incorporation of these actives helped to improve all the parameters evaluated (805 mm
2, 72% and 100.3%, respectively). Helenas et al. [
21] developed a new biocosmetic gel-anionic type containing LEV, and, using the Simple Lattice Design, they evaluated the optimized formulation, which was composed of LEV (1%), avocado oil (0.9 mL) and aloe vera extract (0.1 mL). According to statistical analyses, this formulation would have good spreadability (767.30 mm
2), antioxidant capacity (76%) and moisturizing capacity (98.37%). All studies have shown that LEV has useful properties as active ingredients in cosmetics.
SOPs correspond to biosurfactants, which consist of disaccharide sophoroses (2′-O-β-D-glucopyranosyl-1-β-D-glucopyranose) linked by glycosidic bonds to a fatty acid chain [
22], which are obtained from non-pathogenic fungal strains such as
Starmerella bombicola. These have moisturizing, antioxidant and antimicrobial properties, which have high applicability in the cosmetic industry [
22,
23,
24,
25,
26]. Maeng et al. [
27] produced SOP from
Candida bombicola using horse oil, and they verified its properties. This biomolecule presented the capacity of expressing collagen I and helped in fibroblast migration in human skin. Gaur et al. [
23] evaluated the antimicrobial capacity of SOP and showed that this biosurfactant presented activity against
Pseudomonas aeruginosa,
Escherichia coli,
Bacillus subtilis and
Staphylococcus aureus by destabilizing the permeability of bacterial cytoplasmic membranes. Filipe et al. [
26] developed a self-preserving cosmetic formulation containing SOP (0.4 g) and palmarosa essential oil (0.04 g) that showed action against acne-causing microorganisms. Costa et al. [
25] developed a multifunctional lipstick, containing SOP (1 g) and palmarosa essential oil (0.2 g), due to the antioxidant activity presented by SOP (59.4%). They also verified that the lipstick with the actives presented better spreadability (201.5 mm
2), moisture-retention capacity (91.57%), occlusive factor (85.6) and fusion (63 °C) and breaking (89 g) points when compared to the formulation without the actives (179.1 mm
2, 90.83%, 80.47, 59 °C and 85 g, respectively). Many studies have shown the potential of SOPs as cosmetic ingredients.
The use of essential oils (EO) by the cosmetic industry has stood out [
28], both due to their aroma and the various bioactive and pharmacological properties they present [
29].
Citrus paradisi (grapefruit) essential oil (OCP), which is recognized as GRAS (Generally Recognized as Safe by the FDA—Food and Drug Administration), has several bioactive properties, such as antimicrobial, which can be applied in the development of cosmetic products [
30].
The quality of a cosmetic product involves its effectiveness and safety of use, the stability of the formula and the sensorial aspect. In order to evaluate formulation effects, the biophysical study of the skin has been widely used, as it allows the application of methods that evaluate skin characteristics, such hydration and oiliness,
in vivo through non-invasive, fast and safe techniques through impedance or capacitance methods [
31]. The bioimpedance method consists of passing an electrical current of low intensity (500 to 800 μÄ) and high frequency (50 kHz) through the labial region (which appears as conductive). With the resistance of this conductor to the passage of electrical current, the results of the analysis will be obtained through percentages for skin hydration and oiliness. The Skin Analyzer Digital equipment is based on this method [
32]. The Corneometer® equipment is based on the capacitance method, with an acoustic signal triggered due to electromagnetic contact, and it is cited in the literature as a sensitive instrument for water content measurements [
33]. In addition to the methods presented, other techniques can be used for lip evaluation, such as clinical and histological analyses to investigate lip healing after treatment [
34]. Apart from proving the clinical efficacy presented by cosmetic products, it is essential that the formulation presents a good sensorial, which implies the well-being of the consumer, the acceptance of the product and its long-term use [
35], with sensorial analysis a useful and relevant tool, which guarantees the quality of products developed considering consumer expectations and the benefits highlighted [
36].
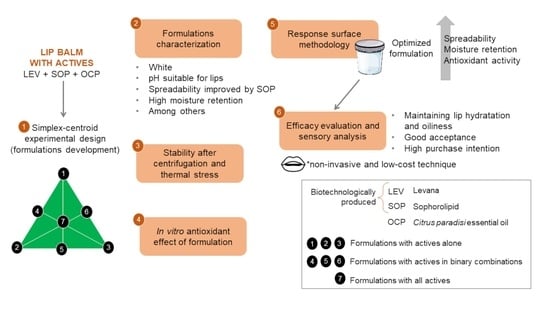
Based on the information presented, the main objective of this work was to develop a multifunctional lip moisturizer as a new biotechnological cosmetic containing LEV from Bacillus subtilis natto, SOP from Starmerella bombicola and OCP as active ingredients, in addition to evaluating the clinical efficacy of the formulation against the parameters of hydration and lip oiliness through a non-invasive technique, as well as evaluating sensory aspects of this, such as intensity of attributes, acceptance and purchase intention. This is an innovative work, which adds knowledge in the field of cosmetology.
4. Discussion
The search and development of natural, sustainable and biodegradable products have emerged in the cosmetic and pharmaceutical markets as an alternative to synthetic compounds. Molecules obtained by biotechnology, such as LEV from
B. subtilis natto and SOP from
S. bombicola, which were obtained through fermentative processes, are examples of active ingredients that meet this trend [
12]. For the first time, a lip moisturizer containing LEV and SOF in combination was developed, resulting in an innovative product.
In this study, the production of LEV was 42.93 g·L
−1, which was higher than that reported by other studies. Pei et al. [
17] described a production of 4.82 g·L
−1 of LEV from
B. megaterium. Bouallegue et al. [
19] reported a production of 2.85 g·L
−1 of LEV from
B. subtilis A17. Gojgic-Cvijovic et al. [
52] reported a production of LEV by
B. licheniformis NS032 between 6.53 and 52.85 g·L
−1, varying according to the fermentative parameters. All these studies produced LEV by the microorganism and not by the enzyme. LEV production in the present study was high due to previous studies carried out by our research group related to fermentative parameters [
53,
54] and the use of the enzyme levansucrase for its production. Other studies that used levansucrase for the production of LEV presented higher production than those that used only the microorganism, such as reported by Ko et al. [
55], with a production of 76 g·L
−1, and Wang et al. [
56], with a production of 30.6 g·L
−1. As seen, enzymes derived from microorganisms are important tools in the biotechnological process, being more useful than naturally occurring enzymes in microorganisms [
57], probably because in microbial cells there are other enzymatic routes that interfere in the best yield of the levansucrase route.
SOP production was 87.10 g·L
−1, which was superior to that described by other studies. Silveira et al. [
37] reported a production of 69.83 g·L
−1 of SOP from
S. bombicola; Hipólito et al. [
22] showed a production of 67.0 g·L
−1, which was lower than that described by Caretta et al. [
58] who reported SOP production of 111.25 g·L
−1. All these studies used glucose and oleic acid as substrates, and it was shown in previous studies that the use of them allows the production optimization of this biosurfactant [
22,
26,
37,
58]. The production of SOP by Intasit and Soontorngun [
59] using as co-subtracts glucose and palm oil was 27.87–30.78 g·L
−1, while the production of SOP by Kim et al. [
60] was 24.1 g·L
−1 using as substrates glucose, rapeseed oil, ammonium nitrate and yeast extract. Both studies presented a lower production of SOP compared to those that used glucose and oleic acid as substrates. Furthermore, glucose and oleic acid favored the production of lactonic SOP, which has been recognized in the literature as a potent antimicrobial agent [
61].
The antimicrobial tests were carried out with the active ingredients SOP and OCP, which have reports in the literature of their antimicrobial properties. The microorganisms used to carry out the analyzes are related to infections caused in the lip region [
62,
63,
64,
65], in addition to being part of the microbiota of this region and the oral cavity. Regarding antimicrobial activity (
Table 1), SOP showed an MIC range of 0.012–0.048 mg·mL
−1 for
S. aureus,
S. epidermidis and
S. mutans, while OCP presented an MIC range of 10.44–41.75 mg·mL
−1 for the same microorganisms. Da Fontoura et al. [
66] reported that SOPs from
S. bombicola presented an MIC value of 500 μg·mL
−1 for
S. aureus ATCC 6336 and
S. mutans ATCC 25175, while Filipe et al. [
26] found that SOPs from
S. bombicola presented an MIC of 31.25 μg·mL
−1 for
S. aureus and 125 μg·mL
−1 for
S. epidermidis. The action of SOP has been reported in other studies [
37,
58,
67,
68]; its antimicrobial activity occurs mainly due to destabilization or alteration of the cell membrane of pathogens, which leads to changes in its permeability, inducing loss of cytoplasmic content and, consequently, death. This antimicrobial mechanism of SOPs is related to their surfactant effect caused by the amphiphilic nature of their molecule, which allows interactions to occur between the sugar (sophorose) and the lipid portion, resulting in damage to the bacterial envelope. SOPs have action against Gram-negative and Gram-positive, but their effect is more noticeable against this last bacterial group, which indicates that SOP antimicrobial action is influenced by the composition of the bacterial cell wall [
26,
37,
58,
66].
In this study, OCP showed lower antimicrobial activity than SOP against
S. aureus,
S. epidermidis and
S. mutans once OCP MIC values were higher than SOP MIC values. Denkova-Kostova et al. [
69] reported an OCP MIC of 6 ppm (0.006 mg·mL
−1) against
S. aureus, while Deng et al. [
70] showed a value of 6.25 µL·mL
−1 (5.21 mg·mL
−1). Filoche, Soma and Sissons [
71] reported that OCP MIC was greater than 10 mg·mL
−1 against
S. mutans. The effect of OCP against various microorganisms has already been proven by several studies [
61,
72,
73,
74]. The antimicrobial property is related to the chemical composition (secondary metabolites) and hydrophobicity of the essential oil. Limonene is the most abundant component in OCP, as well as in most essential oils from
Citrus species, which is related to its antibacterial and antifungal activities. Flavonoids and phenolic compounds may help the antimicrobial effect of limonene. The hydrophobicity allows the essential oil to interact with the bacterial cell membrane, causing changes in this structure that make it more permeable, leading to loss of cytoplasmic material and cell death [
61,
69,
72,
73].
In addition to the antimicrobial effect, OCP is described in the literature for its antioxidant activity, which varies according to the extraction method used to obtain the oil. Denkova-Kostova et al. [
69] reported that the OCP obtained by distillation had an antioxidant potential of 87.5% at a concentration of 1.0 mg·mL
−1, according to DPPH assay. Ou et al. [
75] reported that OCP obtained by distillation had better antioxidant potential (51.24%, at a concentration of 40 mg·mL
−1) than OCP oil obtained by cold pressing (7.75%, at the same concentration). Based on a DPPH test, Lin et al. [
76] reported that OCP obtained by cold compression presented antioxidant activity of 6.3% at a concentration of 5.0 mg·mL
−1. Yang et al. [
77] reported in their study that the OCP showed low antioxidant potential (18.3%, DPPH assay) at a concentration of 5.0 mg·mL
−1. Essential oils rich in monoterpenes (limonene and α-pinene), such as OCP, have significant antioxidant activity due to the fact that these secondary metabolites are oxygenated monoterpenes, which have strongly active methylene groups in their molecule [
54,
75]. In our study, OCP did not show significant antioxidant activity, which was concentration-dependent. The low antimicrobial activity and the lack of antioxidant activity observed in our study may have occurred due to factors that influenced the OCP’s composition, which is fundamental for those activities, like the extraction method, which was cold pressing, the part of the plant used, the vegetative age and the origin of the plant [
61,
73].
The SOP obtained in our study presented a medium antioxidant potential, which was concentration-dependent. The antioxidant activity of SOPs is poorly described in the literature. Filipe et al. [
26] reported that SOP obtained from
S. bombicola presented low antioxidant potential (28.31%) at a concentration range of 2.0–6.0 mg·mL
−1. Kumari et al. [
78] demonstrated in their study the antioxidant activity of SOPs (at 10 mg·mL
−1) from
Metschnikowia churdharensis, which was 62.98%. Costa et al. [
25] showed that SOP (at 10 mg·mL
−1) obtained from
Starmerella bombicola had an antioxidant capacity of 59.40%, based on DPPH assay. Antioxidant activity of SOPs is due to their ability to donate hydrogens and stabilize free radicals such as DPPH [
78]. This antioxidant action may help delay skin aging, which is strongly related to the cumulative effect of oxidative damage [
26].
Several authors have already described in the literature the antioxidant activity of levan based on DPPH assay. Pei et al. [
17] reported in their study that LEV from
Bacillus megaterium PFY-147 presented antioxidant activity of 35.34% and 94.78% at 0.5 mg·mL
−1 and 5.0 mg·mL
−1, respectively; Srikanth et al. [
40] showed that LEV from
Acetobacter xylinum NCIM2526 presented antioxidant activity of 81.26% at 1.0 mg·mL
−1. Domżał-Kędzia et al. [
18] reported that LEV from
B. subtilis natto KB1 presented antioxidant activity of 31.70% at 0.1 mg·mL
−1. The antioxidant property presented by exopolysaccharides depends on their structural factors, such as their molecular weight, monosaccharide content and the configuration of glycosidic bonds [
19]. The antioxidant activity of LEV may be related to the presence of many hydroxyl groups in its structure, which can react with free radicals and generate chain reactions [
79,
80]. In our study, the antioxidant activity presented by LEV was medium and concentration-dependent.
The choice of concentration of active ingredients (variables) used in experimental planning was based on data of antioxidant and antimicrobial analysis and information from the literature. OCP did not present relevant antioxidant activity at the tested concentrations. The OCP MIC values were very high (above 10.44 mg·mL
−1 or above 5.22 mg·mL
−1 when alone or combined withSOP, respectively). However, as the oil would be applied to a lip product, OCP at 0.3% (2.5 mg·mL
−1) was chosen to avoid undesirable taste and odor. To reduce the risk of allergic reactions, the highest concentration of essential oil used in cosmetics is usually about 2% [
81]. SOP demonstrated excellent antimicrobial activity against the tested microorganisms, with the highest MIC value of 0.048 mg·mL
−1 (0.0048%). In general, the active ingredient is incorporated into cosmetic products at a concentration ten times greater than the minimum concentration of its activity, generally to guarantee its effectiveness in the formulation (i.e., 0.048%). However, as SOP showed no toxicity at concentrations up to 25 mg·mL
−1 (2.5%) and its antioxidant activity was close to 40% at 10 mg·mL
−1 (1.0%), the concentration of SOP chosen to be used in the lip balm was 1%. In addition, our research group previously developed another product containing SOP at 1% [
25]. LEV showed medium antioxidant activity; it did not show any difference in terms of DPPH radical scavenging at 1.0% and 2.0% (33.63 and 34.37%, respectively). LEV also has excellent moisturizing activity [
15], which is similar to the hyaluronic acid effect. As LEV is not cytotoxic [
18] and there are no contraindications of concentrations for its use, it was decided to use 2% of it in the formulations.
The eight formulations developed based on simplex-centroid experimental design were subjected to pharmacotechnical characterizations and remained stable in relation to the analyzed parameters, as shown in
Table 3. The incorporation of SOP in formulations F2, F4, F5 and F7 statistically improved (
p < 0.05) their spreadability in comparison to the base. The formulations F1, F3 and F6 did not show statistically significant differences in terms of spreadability compared to the base. SOPs are formed by a hydrophilic portion (sophorose) and a hydrophobic tail, so they are biomolecules capable of modifying the physicochemical characteristics of formulations, such as spreadability, by reducing the surface and interfacial tension of the system, increasing dissolution of hydrocarbons and facilitating the solubilization and absorption of compounds [
68]. For moisture retention, F4 was the only formulation to present a significant statistical difference (
p < 0.05) compared to FB. All formulations showed excellent moisture retention capacity, which can help in maintaining moisture and hydration levels of the labial SC. After carrying out the pharmacotechnical characterizations, we subjected all formulations to preliminary stability testing over a period of 15 days; they remained stable after being subjected to stress conditions.
The study formulations were also subjected to the antioxidant test by scavenging the DPPH radical. BHT, which is an excellent antioxidant, was not incorporated into the formulations subjected to the DPPH test. As can be seen, even FB showed good antioxidant capacity, which is quite unusual (
Table 4); they contained emollients of natural origin, such as shea butter and castor oil, which have antioxidant properties due to the presence of tocopherols, carotenoids and phenolic compounds in their composition [
82,
83]. The use of the active ingredients SOP, LEV and OCP as antioxidant agents did not change the antioxidant capacity of the formulations compared to FB.
This study optimized the formulation employing response surface methodology (RSM) (
Table 6). Most of the studies available in the literature about the development of lip cosmetic products do not employ statistical tools to assist in optimizing the formulation [
84,
85,
86,
87], as demonstrated in this manuscript. Some exceptions are the study developed by Kamairudin et al. [
88], in which the authors optimized the production of a lipstick based on Pitaya seed oil using D-optimal mixture design, and the study conducted by Poomanee et al. [
89], in which they optimized the formulation of colored lipstick using factorial experimental design. The RSM corresponds to mathematical and statistical techniques that are used in the development of relationships between a response of interest and the variables studied, which may help in the optimize the process by reducing the number of test formulations developed, reducing the number of experiments carried out to test the effectiveness of the product under development, reducing the cost of raw materials and making product development faster, among others [
90,
91].
The spreading capacity of a product is related to the area that it covers when spread during its application on the skin [
92]. According to
Table 6 (item 3.8), tests 1, 3 and 6 (containing LEV alone, OCP alone or a combination of both, respectively) showed low spreading capacity compared to the other tests containing SOP, which showed excellent response for this parameter. Assay 5 (containing both OCP and SOP) showed the highest spreadability value. The incorporation of SOP in formulations helped to improve the spreading capacity of the formulations, as this biosurfactant has the ability to reduce the surface and interfacial tension of the system, modifying its physicochemical characteristics [
68]. The response surface and the profile of prediction value and desirability are presented in
Figure 3. According to statistical analyses, the formulation composed of 0.5 of SOP (0.5%) and 0.5 of OCP (0.15%), without LEV, would be ideal to obtain the lip balm showing the best spreadability. In fact, the incorporation of SOP and OCP could improve this parameter, as they are substances composed of hydrophobic portions or in their entirety, as is the case of OCP, which would act by reducing the interfacial and surface tension of the system, in addition to being active emollients, facilitating the spreading of the formulations. In general, emollients have a great impact on the physical-chemical characteristics of products, such as spreadability; they reduce the formulation’s coefficient of friction, modifying its performance during spreading, in addition to influencing its final consistency [
93].
According to
Table 6, all formulations showed good antioxidant capacity; trial 2 (containing only SOP) showed the best response (53.28%) for this parameter. In this study, SOPs presented low antioxidant activity compared to the literature; however, our research group has already carried out studies showing 59.40% inhibition of the DPPH radical by these biomolecules at 10 mg·mL
−1 [
25]. This property can be attributed to the fact that SOPs donate hydrogens to reactive species, stabilizing them [
78]. The response surface and the profile of prediction value and desirability are presented in
Figure 4. According to statistical analyses, the formulation composed of 0.25 (0.5%) of LEV and 0.75 of SOP (0.75%), with no OCP, would be ideal to obtain the lip balm showing the best antioxidant activity. In fact, the incorporation of LEV and SOP could increase this response, as their antioxidant properties are already described in the literature [
17,
18,
25,
40].
According to data presented in
Table 6, all formulations showed good moisture retention (above 95%); tests 1 (containing only LEV) and 5 (containing SOP and OCP) showed the best response (97.70% and 97.71%, respectively) for this parameter. The moisturizing effect presented by LEV has already been studied by some authors; due to the hydrogen bonds present in its molecule, LEV can retain a vast amount of water, presenting moisturizing activity similar to that of hyaluronic acid [
15]. SOPs are biosurfactants that also have potential effects on skin, especially in terms of hydration; these biomolecules can maintain skin functions due to their lipid portion, which allows their greater penetration into the skin [
94]. The association of SOP with OCP showed good moisture retention. According to statistical analyses, the formulation without levan and composed of 0.5 of sophorolipid (0.5%) and 0.5 OCP (0.15%) would be ideal to obtain the lip balm showing the best moisture retention.
Based on the results obtained for the response surface analysis, it was possible to predict the optimized formulation of the study, which is composed of 0.2 of LEV (0.4%), 0.8 of SOP (0.8%) and without OCP (
Figure 6). As reported in this study, OCP did not show good antioxidant and antimicrobial activities, and it would only be used with the intention of providing fragrance for the formulation, unlike SOP and LEV, which presented slight antioxidant activity and excellent antimicrobial effects. Although OCP helped with spreadability and moisture retention responses when in combination with the other active ingredients, its isolated effect was inferior to SOP and LEV for all responses, in addition to having demonstrated a negative effect when combined with SOP and LEV in the antioxidant activity response (lack of antioxidant activity). In addition to these factors, although OCP is described in the literature as GRAS, some review studies reported it as phototoxic, due to the non-volatile compounds present in its composition, even though the risk is considered low [
30]. In this way, the optimized formulation developed in the study was composed only of LEV and SOP.
Verifying the effectiveness of cosmetic formulations is extremely important, as it involves confirming the claims being proposed by the product, such as aiding hydration, reducing fine lines and retaining oil, among others. Several techniques can be used with this objective, among them, non-invasive biophysical analysis through instrumental evaluation, which are safe, do not harm the participants’ skin and can simulate situations of real use of the formulation [
95], in addition to being an alternative to animal efficacy studies [
96]. The present manuscript shows a clinical study in which lip hydration and oiliness were checked, for the first time, using non-invasive, cheap and portable Skin Analyzer Digital equipment (SkinUp
® Devices), which is based on the bioimpedance method. It is highly sensitive equipment that shows a good correlation with the Corneometer
®, which is widely used for skin hydration analyses, and demonstrates good data reproducibility [
32], being efficient for verifications such as those proposed in this study.
According to the results described in
Table 2, it is possible to observe that participants in G1 and G2 had good lip hydration before application of lip balm (time 0), showing hydration levels above 41%, which are described by the Heinrich score [
97] as normal. The same was true for oiliness, whose levels were above 28%. After 7 days of applying the FT and FB formulations, there was a slight increase in lip hydration, whose levels were close to 45%; however, there was no statistical difference compared to time 0. Oiliness was reduced, maintaining its level close to 25%, but no statistical significance was found either in comparison to time 0. When comparing G1 and G2, after applying the lip balms, there was no statistical difference in the hydration and oil content of participants’ lips; both measurements were very close. No adverse effects or irritability were described by participants throughout the study.
Some factors influenced negatively on results found in this study, such as the limited number of formulations’ daily applications, as described by several participants, which allowed periods of dryness to occur throughout the day, because of the ingestion of food/drinks and the non-reapplication of the product and the temperature changes in the months of April and May (from 30 °C to 15 °C, for example) that favored dry lips, with the appearance of cracks and wounds. To overcome these problems, a greater number of daily applications would be ideal, such as three to four, which would allow the product to form a protective barrier on the lips throughout the day. However, even with these events, it is possible to observe that the use of the formulations developed in this study helped to maintain the lip hydration and oiliness already exhibited by the participants, which is a very promising result.
Studies using the Skin Analyzer Digital device as a technique for evaluating lip hydration and oiliness are not described in the literature, as it is a recent method. In this way, the present study may help in the development of future studies in the field of cosmetology on lip hydration and evaluation of the effectiveness of lip cosmetics using a portable, cheap, sensitive and reproducibility device like the one from SkinUp
® Beauty Device. There are reports in the literature involving other instrumental methods in studies of the effectiveness of lip products, such as the Corneometer
®, which is a non-portable and more expensive device; Gfeller et al. [
1] developed a lip cream containing micro repair technology that improved dryness compared to the untreated group; Bielfeldt et al. [
3] developed a lip cosmetic containing natural emollients that improved hydration, as well as reducing transepidermal water loss from the lips. Furthermore, there are no studies in the literature that report the development of lip products containing LEV and SOP in combination, which makes the cosmetics developed in this study innovative.
Sensory analysis is a useful and highly important tool in the cosmetic industry, as it helps in the development of products, ensuring their quality, and in aggregate marketing, in addition to allowing the evaluation of product acceptance among the consumer public [
98]. In the present study, untrained evaluators but potential consumers of lip products participated in the development of sensory analyses, which can be verified through
Figure 9; 50% of these participants used it daily, several times a day, 17% of participants used lip balms daily, once a day and 7% used lip balms weekly.
According to the intensity of the attributes (
Table 3), it is possible to verify that there was no significant statistical difference between FT and FB for all parameters evaluated, with values above 6.2 (tending to “very intense”), with the exception of fragrance, which presented values close to 4 (“neither too intense nor too little intense”) due to the non-incorporation of essential oils or aromas, maintaining the characteristic odor of the formulations. The standard deviations ranged from 1.28 to 2.68, which indicates a great variability among the evaluators’ response; it probably occurred due to the difficulty in describing and discriminating aspects of both formulations, like aroma, spreadability and freshness, that are very similar [
36]. The non-statistical significance obtained in this analysis only confirms the difficulty of differentiating between FB and FT, which demonstrates that the incorporation of LEV and SOP actives does not modify the evaluators’ perception or result in sensory changes in formulation (when comparing FT to the control).
The formulations showed good acceptance rates, which were 84.71% for FT and 78.86% for FB, being qualitatively shown as “I liked” and “I really liked”. The results obtained using the hedonic scale showed statistical significance, demonstrating that there was preference for FT when compared to FB, even with the difficulties faced in differentiating the formulations in relation to their attributes. In fact, the hedonic scale allows evaluators to choose the answers that suit their preferences and that reflect their opinion regarding the products tested, without the need to form a trained panelist group, thus helping in the development of several studies in which it is intended to know a preference sample [
49]. A lip balm development study conducted by Azmin, Jaine and Nor [
85] used a hedonic scale to evaluate the spreadability, color, odor and general acceptance of different samples, comparing the results with previously carried out instrumental analyses. They verified that there was no significant difference between the attributes evaluated, so all the lip balms produced could be commercialized. A study developed by Esposito and Kirilov [
99] used a 9-point hedonic scale to evaluate spreadability, hardness, opacity, gloss effect and oiliness of different lipstick samples. They verified, for example, that greasiness and glossiness presented a significant difference among the formulations, because of the composition of lipsticks (concentration of vaseline), while the spreadability was good for all samples, without significant difference.
The acceptance rates were confirmed through purchase intention, which were 4.087 ± 0.78 for FT and 3.848 ± 0.87 for FB, being qualitatively shown as “maybe I would buy, might not buy” for FB and “probably I would buy” for FT. The probability of consumers purchasing a cosmetic product is mainly determined by its sensoriality; regarding lip products, it is also determined by the sensation felt during application [
100]. All attributes (ease of spreading, absorption, hydration, freshness, formation of a velvety film and fragrance) evaluated in this study were well accepted by the evaluators, which consequently influenced the positive purchase intention of the present lip balm.
After a period of 7 days of applying the FT lip balm daily, a self-assessment test was submitted to the participants to verify the long-term effectiveness of the product [
51]. For improvement in lip dryness and roughness, 85% of participants reported that the formulation helped to hydrate their lips, while 15% did not observe this effect (
Figure 11). This is a promising result, as it demonstrates that the hydration attribute may not be perceived immediately after application; however, over the days, it promotes an effect on dryness and roughness of the lips.
As can be seen in this study, sensory analysis is a tool that assists in the development and evaluation of cosmetic products. Several studies on lip products, such as those carried out by Abidh et al. [
100], Kasparaviciene et al. [
101] and Rafferty et al. [
102], demonstrate the importance of this science in determining and considering the properties and attributes of cosmetics intended for the lips.