Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs
Abstract
:1. Introduction
2. Skin Permeation Pathways
3. Determination of Transdermal Permeability Coefficient
4. NE in Transdermal Delivery
4.1. Physical Properties of Transdermal NE
4.1.1. Oil-in-Water (o/w) NE
4.1.2. Water-in-Oil (w/o) NE
4.2. Components of Transdermal NE
4.2.1. Oil Phase
4.2.2. Surfactants
4.2.3. Co-Surfactants
4.2.4. Permeation Enhancers (Accelerants)
5. Biocidal Property of NE
6. Methods of NE Preparation
6.1. High Energy Methods
6.1.1. High-Pressure Homogenization
6.1.2. Microfluidization
6.1.3. Ultra-Sonication
6.1.4. Jet Disperser
6.2. Low Energy Methods
6.2.1. Phase Inversion Temperature
6.2.2. Spontaneous Emulsification
6.2.3. Solvent Displacement Method
7. Characterization of Transdermal NE
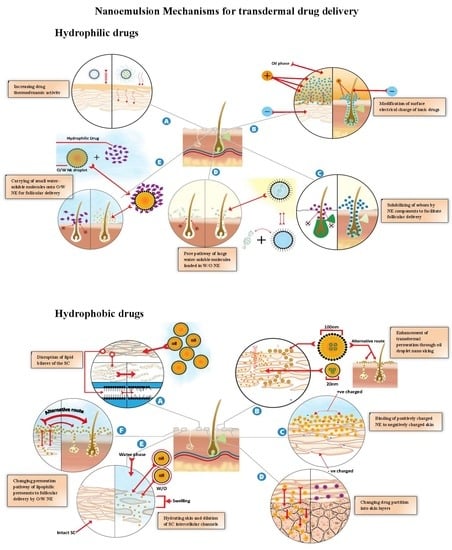
8. NEs for Transdermal Delivery of Hydrophilic Drugs
8.1. Transdermal w/o NEs Containing Hydrophilic Drugs
8.2. NE Mechanisms for Enhanced Transdermal Delivery of Hydrophilic Drugs
8.2.1. Increasing Drug Thermodynamic Activity
8.2.2. Modification of Surface Electrical Charge of Ionic Drugs
8.2.3. Solubilization of Sebum by NE Components
8.2.4. Pore Pathway for the Transport of Large Water-Soluble Molecules Loaded into W/O NEs
8.2.5. Carrying of Small Water-Soluble Molecules into O/W NE for Follicular Delivery
9. NEs for the Transdermal Delivery of Hydrophobic Drugs
9.1. Transdermal O/W NEs Containing Hydrophobic Drugs
9.2. NE Mechanisms for the Enhanced Transdermal Delivery of Hydrophobic Drugs
9.2.1. Disruption of the SC Lipid Bilayers
9.2.2. Enhancement of Transdermal Permeation through the Nano-Sizing of Oil Droplets
9.2.3. Binding of the Positively Charged NE to Negatively Charged Skin
9.2.4. Enhancement Transdermal Permeation by Reducing the NE Viscosity
9.2.5. Changing the Drug Partition into Skin Layers
9.2.6. Hydrating the Skin and Dilating the SC Intercellular Channels
9.2.7. Changing the Permeation Pathway of Lipophilic Permeants to Follicular Delivery
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ALA | 5-aminolevulinic acid |
| CVS | cardiovascular system |
| DHPS | 3,5-Dihydroxy-4-isopropylstilbene |
| DMSO | Dimethyl sulfoxide |
| EIP | emulsion inversion point |
| GIT | gastrointestinal tract |
| HLB | hydrophile-lipophile balance |
| IPA | Isopropyl alcohol |
| IPM | Isopropyl myristate |
| MCZ | Miconazole |
| ME | Microemulsion |
| MLX | Meloxicam |
| MPa | Mega Pascal |
| Mw | molecular weight |
| NE | Nanoemulsion |
| NMP | N-methyl pyrrolidone |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| o/w | oil-in-water |
| OA | Oleic acid |
| PE | Permeation enhancer |
| PEG | Polyethylene glycol |
| PIT | Phase inversion temperature |
| PS | Particle size |
| psi | Pounds square inch |
| RHCL | Ropinirole hydrochloride |
| SC | Stratum corneum |
| SLS | sodium lauryl sulfate |
| Smix | surfactant/co-surfactant mixture |
| TCC | Thiocolchicoside |
| CBX | Celecoxib |
| w/o | water-in-oil |
References
- Thakur, N.; Garg, G.; Sharma, P.K.; Kumar, N. Nanoemulsions: A Review on Various Pharmaceutical Application. Glob. J. Pharmacol. 2012, 6, 222–225. [Google Scholar]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloids Surf. B Biointerfaces 2010, 75, 356–362. [Google Scholar] [CrossRef]
- Çinar, K. A Review on Nanoemulsions: Preparation Methods and Stability. Trak. Univ. J. Eng. Sci. 2017, 18, 73–83. [Google Scholar]
- Sarker, D. Engineering of Nanoemulsions for Drug Delivery. Curr. Drug Deliv. 2005, 2, 297–310. [Google Scholar] [CrossRef]
- Mostafa, D.M.; Kassem, A.A.; Asfour, M.H.; Al Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.S. Transdermal cumin essential oil nanoemulsions with potent antioxidant and hepatoprotective activities: In-vitro and in-vivo evaluation. J. Mol. Liq. 2015, 212, 6–15. [Google Scholar] [CrossRef]
- Khopade, A.J.; Nandakumar, K.S.; Jain, N.K. Lectin-functionalized multiple emulsions for improved cancer therapy. J. Drug Target. 1998, 6, 285–292. [Google Scholar] [CrossRef]
- Hildebrand, A.; Schaedlich, A.; Rothe, U.; Neubert, R.H.H. Sensing specific adhesion of liposomal and micellar systems with attached carbohydrate recognition structures at lectin surfaces. J. Colloid Interface Sci. 2002, 249, 274–281. [Google Scholar] [CrossRef]
- Barakat, N.; Fouad, E.; Elmedany, A. Formulation Design of Indomethacin-Loaded Nanoemulsion For Transdermal Delivery. Pharm. Anal. Acta 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Shafaat, K.; Kumar, B.; Das, S.K.; Ul Hasan, R.; Prajapati, S.K. Novel nanoemulsion as vehicles for transdermal delivery of Clozapine: In vitro and in vivo studies. Int. J. Pharm. Pharm. Sci. 2013, 5, 126–134. [Google Scholar]
- Iman, I.S.; Nadia, A.S.; Ebtsam, M.A. Formulation and stability study of chlorpheniramine maleate transdermal patch. Asian J. Pharm. 2010, 4, 17–23. [Google Scholar]
- Peira, E.; Scolari, P.; Gasco, M.R. Transdermal permeation of apomorphine through hairless mouse skin from microemulsions. Int. J. Pharm. 2001, 226, 47–51. [Google Scholar] [CrossRef]
- Singh, I.; Morris, A.P. Performance of transdermal therapeutic systems: Effects of biological factors. Int. J. Pharm. Investig. 2011, 1, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.E.; Blankschtein, D.; Langer, R. Evaluation of solute permeation through the stratum corneum: Lateral bilayer diffusion as the primary transport mechanism. J. Pharm. Sci. 1997, 86, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Barry, B.W. Lipid-Protein-Partitioning theory of skin penetration enhancement. J. Control. Release 1991, 15, 237–248. [Google Scholar] [CrossRef]
- Roberts, M.S. Targeted drug delivery to the skin and deeper tissues: Role of physiology, solute structure and disease. Clin. Exp. Pharmacol. Physiol. 1997, 24, 874–879. [Google Scholar] [CrossRef]
- Ng, K.W.; Lau, W.M. Skin deep: The basics of human skin structure and drug penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Maibach, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. ISBN 9783662450130. [Google Scholar]
- Shaker, D.S.; Ghanem, A.H.; Li, S.K.; Warner, K.S.; Hashem, F.M.; Higuchi, W.I. Mechanistic studies of the effect of hydroxypropyl-β-cyclodextrin on in vitro transdermal permeation of corticosterone through hairless mouse skin. Int. J. Pharm. 2003, 253, 1–11. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J. Nanobiotechnol. 2008, 6, 8. [Google Scholar] [CrossRef]
- Scheuplein, R.J. Mechanism of Percutaneous Absorption: II. Transient Diffusion and the Relative Importance of Various Routes of Skin Penetration. J. Investig. Dermatol. 1967, 48, 79–88. [Google Scholar] [CrossRef]
- Trauer, S.; Lademann, J.; Knorr, F.; Richter, H.; Liebsch, M.; Rozycki, C.; Balizs, G.; Büttemeyer, R.; Linscheid, M.; Patzelt, A. Development of an in vitro modified skin absorption test for the investigation of the follicular penetration pathway of caffeine. Skin Pharmacol. Physiol. 2010, 23, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Warner, K.S.; Kevin Li, S.; Higuchi, W.I. Influences of alkyl group chain length and polar head group on chemical skin permeation enhancement. J. Pharm. Sci. 2001, 90, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Yoneto, K.; Ghanem, A.-H.; Higuchi, W.I.; Peck, K.D.; Li, S.K. Mechanistic studies of the 1-alkyl-2-pyrrolidones as skin permeation enhancers. J. Pharm. Sci. 1995, 84, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Rees, G.D. Microemulsion-Based Media as Novel Drug Delivery Systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Valenta, C.; Schultz, K. Influence of carrageenan on the rheology and skin permeation of microemulsion formulations. J. Control. Release 2004, 95, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chakravorty, A.; Kumar, P.; Chaturvedi, A. NANOEMULSION: An Effective Therapy for Transdermal Drug Delivery. Res. J. Biol. 2013, 3, 33–38. [Google Scholar]
- Changez, M.; Varshney, M.; Chander, J.; Dinda, A.K. Effect of the composition of lecithin/n-propanol/isopropyl myristate/water microemulsions on barrier properties of mice skin for transdermal permeation of tetracaine hydrochloride: In vitro. Colloids Surf. B Biointerfaces 2006, 50, 18–25. [Google Scholar] [CrossRef]
- Dreher, F.; Walde, P.; Walther, P.; Wehrli, E. Controlled release Interaction of a lecithin microemulsion gel with human. J. Control. Release 1997, 45, 131–140. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Suyal, J.; Ganesh, B. An introductory review article on nanoemulsion. J. Pharm. Pharm. Sci. 2017, 2, 35–40. [Google Scholar]
- Montes de Oca-Ávalos, J.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and physical properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Sharma, N.; Bansal, M.; Visht, S.; Sharma, P.; Kulkarni, G. Nanoemulsion: A new concept of delivery system. Chron. Young Sci. 2010, 1, 2–6. [Google Scholar]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Yoshikawa, T.; Moroto, Y.; Kanaoka, E.; Takahashi, K.; Nishihara, Y.; Masuda, K. Microemulsion formulation for enhanced absorption of poorly soluble drugs: I. Prescription design. J. Control. Release 2002, 81, 65–74. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Benson, H. Transdermal Drug Delivery: Penetration Enhancement Techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Hosmer, J.; Reed, R.; Bentley, M.V.L.B.; Nornoo, A.; Lopes, L.B. Microemulsions Containing Medium-Chain Glycerides as Transdermal Delivery Systems for Hydrophilic and Hydrophobic Drugs. AAPS PharmSciTech 2009, 10, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.; Chen, H.; Weng, T.; Yang, Y.; Yang, X. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur. J. Pharm. Biopharm. 2003, 56, 189–196. [Google Scholar] [CrossRef]
- Pandey, A. Role of Surfactants as Penetration Enhancer in Transdermal Drug Delivery System. J. Mol. Pharm. Org. Process Res. 2014, 2, 2–7. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kasamaki, M.; Ikarashi, A. Effects of n-alkyltrimethylammonium on skin permeation of benzoic acid through excised guinea pig dorsal skin. Chem. Pharm. Bull. 2000, 48, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Scheuplein, R.; Ross, L. Effects of surfactants and solvents on the permeability of epidermis. J. Soc. Cosmet. Chem. 1970, 21, 853–873. [Google Scholar]
- Kouchak, M.; Handali, S. Effects of Various Penetration Enhancers on Penetration of Aminophylline Through Shed Snake Skin. Jundishapur J. Nat. Pharm. Prod. 2014, 9, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.; Guy, R.H.; Hadgraft, J. Prediction of Percutaneous Penetration: Methods, Measurements, Modelling. Scott, R.C., Richard, H., Guy, J.H., Eds.; IBC Technical Services: London, UK, 1990; ISBN 1852711175, 9781852711177. [Google Scholar]
- Breuer, M.M. The interaction between surfactants and keratinous tissues. J. Soc. Cosmet Chem. 1979, 30, 41–64. [Google Scholar]
- Froebe, C.L.; Simion, F.A.; Rhein, L.D.; Cagan, R.H.; Kligman, A. Stratum corneum lipid removal by surfactants: Relation to in vivo irritation. Dermatologica 1990, 181, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lévêque, J.L.; de Rigal, J.; Saint-Léger, D.; Billy, D. How does sodium lauryl sulfate alter the skin barrier function in man? A multiparametric approach. Skin Pharmacol. 1993, 6, 111–115. [Google Scholar] [CrossRef]
- Patil, S.; Singh, P.; Maibach, H. Radial Spread of Sodium Lauryl Sulfate After Topical Application. Pharm. Res. 1995, 12, 2018–2023. [Google Scholar] [CrossRef]
- Patil, S.; Singh, P.; Sarasour, K.; Maibach, H. Quantification of sodium lauryl sulfate penetration into the skin and underlying tissue after topical application—Pharmacological and toxicological implications. J. Pharm. Sci. 1995, 84, 1240–1244. [Google Scholar] [CrossRef]
- Rhein, L.; Robbins, C.; Fernee, K. Surfactant structure effects on swelling of isolated human stratum corneum. J. Soc. Cosmet. Chem. 1986, 139, 125–139. [Google Scholar]
- Gibson, W.T.; Teall, M.R. Interactions of C12 surfactants with the skin: Changes in enzymes and visible and histological features of rat skin treated with sodium lauryl sulphate. Food Chem. Toxicol. 1983, 21, 587–594. [Google Scholar] [CrossRef]
- Thomas, G. Polefka Handbook of detergents Part A: Properties. In Marcel Dekker; Broze, G., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 433–468. ISBN 0-8247-1417-2. [Google Scholar]
- Gradzielski, M. Effect of the Cosurfactant Structure on the Bending Elasticity in Nonionic Oil-in-Water Microemulsions. Langmuir 1998, 14, 6037–6044. [Google Scholar] [CrossRef]
- Zana, R. Surfactant solutions: New methods of Investigation. In Surfuctant Solutions: New Methods of Investigation; Marcel Dekker Inc.: New York, NY, USA, 1979; pp. 2–51. ISBN 0824776232. [Google Scholar]
- Yadav, S.A.; Singh, D.; Poddar, S. Influence of components of nanoemulsion system for transdermal drug delivery of nimodipine. Asian J. Pharm. Clin. Res. 2012, 5, 209–214. [Google Scholar]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Tenjarla, S. Microemulsions: An overview and pharmaceutical applications. Crit. Rev. Ther. Drug Carrier Syst. 1999, 16, 461–521. [Google Scholar] [CrossRef] [PubMed]
- Kegel, W.K.; Lekkerkerker, H.N.W. Phase behaviour of an ionic microemulsion system as a function of the cosurfactant chain length. Colloids Surf. A Physicochem. Eng. Asp. 1993, 76, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Klossek, M.L.; Marcus, J.; Touraud, D.; Kunz, W. The extension of microemulsion regions by combining ethanol with other cosurfactants. Colloids Surf. A Physicochem. Eng. Asp. 2013, 427, 95–100. [Google Scholar] [CrossRef]
- Stilbs, P. Fourier transform NMR pulsed-gradient spin-echo (FT-PGSE) self-diffusion measurements of solubilization equilibria in SDS solutions. J. Colloid Interface Sci. 1982, 87, 385–394. [Google Scholar] [CrossRef]
- Resende, K.X.; Corrêa, M.A.; Gomes De Oliveira, A.; Scarpa, M.V.; Scarpa, M.V. Effect of cosurfactant on the supramolecular structure and physicochemical properties of non-ionic biocompatible microemulsions. Rev. Bras. Ciências Farm. Braz. J. Pharm. Sci. 2008, 44, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Yu, M.; Guo, F.; Li, N.; Tan, F.P. Synergistic effect of mixed cosurfactants on transdermal delivery of indomethacin from O/W microemulsion. Chem. Res. Chin. Univ. 2013, 29, 338–343. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- dos Anjos, J.L.V.; Alonso, A. Terpenes increase the partitioning and molecular dynamics of an amphipathic spin label in stratum corneum membranes. Int. J. Pharm. 2008, 350, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Benson, H.A.E.; Roberts, M.S.; Grice, J.E. Minoxidil skin delivery from nanoemulsion formulations containing eucalyptol or oleic acid: Enhanced diffusivity and follicular targeting. Pharmaceutics 2018, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Niazy, E.M. Influence of oleic acid and other permeation promoters on transdermal delivery of dihydroergotamine through rabbit skin. Int. J. Pharm. 1991, 67, 97–100. [Google Scholar] [CrossRef]
- Mirejovsky, D.; Takruri, H. Dermal penetration enhancement profile of hexamethylenelauramide and its homologues: In vitro versus in vivo behavior of enhancers in the penetration of hydrocortisone. J. Pharm. Sci. 1986, 75, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Madison, K.C. Barrier Function of the Skin: ‘“La Raison d’Etre”’ of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Aungst, B.J.; Nancy, J.R.; Shefter, E. Enhancement of naloxone penetration through human skin in vitro using fatty acids, fatty alcohols, surfactants, sulfoxides and amides. Int. J. Pharm. 1986, 33, 225–234. [Google Scholar] [CrossRef]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of terpenes as skin penetration enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef]
- Patel, M.; Joshi, A.; Hassanzadeth, H.; Juluru, R.; Stagni, G. Quantification of dermal and transdermal delivery of meloxicam gels in rabbits. Drug Dev. Ind. Pharm. 2011, 37, 613–617. [Google Scholar] [CrossRef]
- Kumar, B.; Jain, S.K.; Prajapati, S.K. Effect of penetration enhancer DMSO on in-vitro skin permeation of acyclovir transdermal microemulsion formulation. Int. J. Drug Deliv. 2011, 3, 83–94. [Google Scholar] [CrossRef]
- Warner, K.S.; Shaker, D.S.; Molokhia, S.; Xu, Q.; Hao, J.; Higuchi, W.I.; Li, S.K. Silicone Elastomer Uptake Method for Determination of Free 1-Alkyl-2-Pyrrolidone Concentration in Micelle and Hydroxypropyl-β-Cyclodextrin Systems Used in Skin Transport Studies. J. Pharm. Sci. 2008, 97, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.R. Effect of Decylmethyl Sulfoxide on Skin Penetration. In Solution Behavior of Surfactants; Springer: Boston, MA, USA, 1982; pp. 1505–1516. [Google Scholar]
- Lee, P.J.; Langer, R.; Shastri, V.P. Novel microemulsion enhancer formulation for simultaneous transdermal delivery of hydrophilic and hydrophobic drugs. Pharm. Res. 2003, 20, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Sharma, P.; Irchhiaya, R. Effect of alcohols and enhancers on permeation enhancement of ketorolac. Asian J. Pharm. 2009, 3, 37. [Google Scholar] [CrossRef]
- Pershing, L.K.; Lambert, L.D.; Knutson, K. Mechanism of ethanol-enhanced estradiol permeation across human skin in vivo. Pharm. Res. 1990, 7, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.H.; Mahmoud, H.; Higuchi, W.L.; Rohr, U.D.; Borsadia, S.; Liu, P.; Fox, J.L.; Good, W.R. The effects of ethanol on the transport of β-estradiol and other permeants in hairless mouse skin. II. A new quantitative approach. J. Control. Release 1987, 6, 75–83. [Google Scholar] [CrossRef]
- Ghanem, A.H.; Mahmoud, H.; Higuchi, W.I.; Liu, P.; Good, W.R. The effects of ethanol on the transport of lipophilic and polar permeants across hairless mouse skin: Methods/validation of a novel approach. Int. J. Pharm. 1992, 78, 137–156. [Google Scholar] [CrossRef]
- Myc, A.; Vanhecke, T.; Landers, J.J.; Hamouda, T.; Baker, J.R. The fungicidal activity of novel nanoemulsion (X8W60PC) against clinically important yeast and filamentous fungi. Mycopathologia 2002, 155, 195–201. [Google Scholar] [CrossRef]
- Rasouli, R.; Alaei-Beirami, M.; Zaaeri, F. Nanobiomaterials Applications in Drug Delivery; Sharma, K.A., Keservani, K.R., Kesharwani, K.R., Eds.; Apple Academic Press: Cambridge, MA, USA, 2018; ISBN 9781351792455. [Google Scholar]
- Hamouda, T.; Hayes, M.M.; Cao, Z.; Tonda, R.; Johnson, K.; Wright, D.C.; Brisker, J.; Baker, J.R. A Novel Surfactant Nanoemulsion with Broad-Spectrum Sporicidal Activity against Bacillus Species. J. Infect. Dis. 1999, 180, 1939–1949. [Google Scholar] [CrossRef]
- Wright, D.C. Antibacterial Oil-in-Water Emulsions. U.S. Patent 5,618,840, 1997. [Google Scholar]
- Shah, P.; Bhalodia, D.; Shelat, P. Nanoemulsion: A pharmaceutical review. Syst. Rev. Pharm. 2010, 1, 24–32. [Google Scholar] [CrossRef]
- Hamouda, T.; Myc, A.; Donovan, B.; Shih, A.Y.; Reuter, J.D.; Baker, J.R. A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol. Res. 2001, 156, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jasmina, H.; Džana, O.; Alisa, E.; Edina, V.; Ognjenka, R. Preparation of nanoemulsions by high-energy and lowenergy emulsification methods. In CMBEBIH 2017. IFMBE Proceedings; Badnjevic, A., Ed.; Springer: Singapore, 2017; Volume 62, pp. 317–322. [Google Scholar]
- Hussan Reza, K. Nanoemulsion as a novel transdermal drug delivery system. Int. J. Pharm. Sci. Res. 2011, 2, 1938–1946. [Google Scholar]
- Brewer, E.T. A comparative evaluation of indomethacin, acetaminophen and placebo as antipyretic agents in children. Arthritis Rheum. 1968, 11, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; John, A. Nano-Emulsion in Pharmaceuticals: A Review. Curr. Res. Drug Target 2015, 5, 1–4. [Google Scholar]
- Leong, T.S.H.; Wooster, T.J.; Kentish, S.E.; Ashokkumar, M. Minimising oil droplet size using ultrasonic emulsification. Ultrason. Sonochem. 2009, 16, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinoda, K.; Saito, H. The effect of temperature on the phase equilibria and the types of dispersions of the ternary system composed of water, cyclohexane, and nonionic surfactant. J. Colloid Interface Sci. 1968, 26, 70–74. [Google Scholar] [CrossRef]
- Maali, A.; Mosavian, M.T.H. Preparation and Application of Nanoemulsions in the Last Decade (2000–2010). J. Dispers. Sci. Technol. 2013, 34, 92–105. [Google Scholar] [CrossRef]
- Yukuyama, M.; Kato, E.; Löbenberg, R.; Bou-Chacra, N. Challenges and future prospects of nanoemulsion as a drug delivery system. Curr. Pharm. Des. 2016, 22, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Anton, N.; Saulnier, P. Adhesive water-in-oil nano-emulsions generated by the phase inversion temperature method. Soft Matter 2013, 9, 6465–6474. [Google Scholar] [CrossRef] [Green Version]
- Forgiarini, A.; Esquena, J.; González, C.; Solans, C. Formation of nano-emulsions by low-energy emulsification methods at constant temperature. Langmuir 2001, 17, 2076–2083. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates-A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef]
- Basha, S.P.; Rao, K.P.; Vedantham, C. A brief introduction to methods of preparation, applications and characterization of nanoemulsion drug delivery systems. Indian J. Res. Pharm. Biotechnol. 2013, 1, 25–28. [Google Scholar]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Majithiya, R.J.; Umrethia, M.L.; Murthy, R.S.R. Design and development of microemulsion drug delivery system of acyclovir for improvement of oral bioavailability. AAPS PharmSciTech 2006, 7, E172. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.Q.M.; Barker, S.A.; Banning, D.; Booth, S.W. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int. J. Pharm. 1995, 114, 103–110. [Google Scholar] [CrossRef]
- Halnor, V.; Pande, V.; Borawake, D.; Nagare, H. Nanoemulsion: A Novel Platform for Drug Delivery System. J. Mater. Sci. Nanotechnol. 2018, 6, 104–115. [Google Scholar]
- Chiesa, M.; Garg, J.; Kang, Y.T.; Chen, G. Thermal conductivity and viscosity of water-in-oil nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 326, 67–72. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Amemiya, F.; Fuchigami, T.; MacHida, K.; Takeda, S.; Tamamitsu, K.; Atobe, M. Highly clear and transparent nanoemulsion preparation under surfactant-free conditions using tandem acoustic emulsification. Chem. Commun. 2011, 47, 5765–5767. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.S.; Kong, B.J.; Cho, W.G.; Park, S.N. Formation of stable hydrocarbon oil-in-water nanoemulsions by phase inversion composition method at elevated temperature. Korean J. Chem. Eng. 2015, 32, 540–546. [Google Scholar] [CrossRef]
- Mason, T.G.; Graves, S.M.; Wilking, J.N.; Lin, M.Y. Extreme emulsification: Formation and structure of nanoemulsions. Condens. Matter Phys. 2006, 9, 193–199. [Google Scholar] [CrossRef]
- Samah, N.A.; Williams, N.; Heard, C.M. Nanogel particulates located within diffusion cell receptor phases following topical application demonstrates uptake into and migration across skin. Int. J. Pharm. 2010, 401, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Preetz, C.; Hauser, A.; Hause, G.; Kramer, A.; Mäder, K. Application of atomic force microscopy and ultrasonic resonator technology on nanoscale: Distinction of nanoemulsions from nanocapsules. Eur. J. Pharm. Sci. 2010, 39, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Anton, N.; Ta, T.M.C.; Zhao, M.; Messaddeq, N.; Vandamme, T.F. Microencapsulation of nanoemulsions: Novel Trojan particles for bioactive lipid molecule delivery. Int. J. Nanomed. 2011, 6, 1313–1325. [Google Scholar]
- Khurana, S.; Jain, N.K.; Bedi, P.M.S. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sci. 2013, 92, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Saint Ruth, H.; Attwood, D.; Ktistis, G.; Taylor, C.J. Phase studies and particle size analysis of oil-in-water phospholipid microemulsions. Int. J. Pharm. 1995, 116, 253–261. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.H.; Hussein, A.A. Oral nanoemulsions of candesartan cilexetil: Formulation, characterization and in vitro drug release studies. AAPS Open 2017, 3, 4. [Google Scholar] [CrossRef]
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Formulation of nanoemulsion: A comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015, 22, 455–466. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; Elnaggar, Y.S.R.; Gohar, E.Y.; Abdallah, O.Y. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: Optimization and in vivo appraisal. Int. J. Nanomed. 2012, 7, 3787–3802. [Google Scholar]
- Debnath, S.; Satayanarayana Kumar, G.V. Nanoemulsion-a method to improve the solubility of lipophilic drugs. Pharmanest 2011, 2, 72–83. [Google Scholar]
- Ganta, S.; Sharma, P.; Paxton, J.W.; Baguley, B.C.; Garg, S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in long-circulating nanoemulsion. J. Drug Target 2010, 18, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of triptolide-nanoemulsion gels for percutaneous administration: Physicochemical, transport, pharmacokinetic and pharmacodynamic characteristics. J. Nanobiotechnol. 2017, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Shahtalebi, M.A.; Sadat-Hosseini, A.; Safaeian, L. Preparation and evaluation of clove oil in emu oil self-emulsion for hair conditioning and hair loss prevention. J. HerbMed Pharmacol. 2016, 5, 72–77. [Google Scholar]
- Kurup, N.S.; Joshi, P.R. Formulation and evaluation of herbal microemulsion for controlling hair loss. Int. J. Res. Pharm. Sci. 2013, 4, 420–426. [Google Scholar]
- Hu, L.; Hu, Q.; Yang, J. Enhancement of transdermal delivery of ibuprofen using microemulsion vehicle. Iran. J. Basic Med. Sci. 2014, 17, 760–766. [Google Scholar] [PubMed]
- Joshi, M.; Pathak, S.; Sharma, S.; Patravale, V. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: Nanoject. Int. J. Pharm. 2008, 364, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.C.; Klang, V.; Karall, S.; Mahrhauser, D.; Resch, G.P.; Valenta, C. Optimisation of multiple W/O/W nanoemulsions for dermal delivery of aciclovir. Int. J. Pharm. 2012, 435, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Andalib, S.; Tabbakhian, M.; Ebrahimzadeh, N. Development of lecithin nanoemulsion based organogels for permeation enhancement of metoprolol through rat skin. J. Nanomater. 2013, 2013, 6. [Google Scholar] [CrossRef]
- Tsai, M.J.; Fu, Y.S.; Lin, Y.H.; Huang, Y.B.; Wu, P.C. The effect of nanoemulsion as a carrier of hydrophilic compound for transdermal delivery. PLoS ONE 2014, 9, e102850. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.W.; Ward, A.J.; O'NEILL, K.J. Microemulsions as topical drug delivery vehicles: In-vitro transdermal studies of a model hydrophilic drug. J. Pharm. Pharmacol. 1991, 43, 451–454. [Google Scholar] [CrossRef]
- Raza, K.; Negi, P.; Takyar, S.; Shukla, A.; Amarji, B.; Katare, O.P. Novel dithranol phospholipid microemulsion for topical application: Development, characterization and percutaneous absorption studies. J. Microencapsul. 2011, 28, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ramachandran, C.; Weiner, N.D.; Roessler, B.J. Topical transport of hydrophilic compounds using water-in-oil nanoemulsions. Int. J. Pharm. 2001, 220, 63–75. [Google Scholar] [CrossRef]
- Kattou, P.; Lian, G.; Glavin, S.; Sorrell, I.; Chen, T. Development of a Two-Dimensional Model for Predicting Transdermal Permeation with the Follicular Pathway: Demonstration with a Caffeine Study. Pharm. Res. 2017, 34, 2036–2048. [Google Scholar] [CrossRef]
- Elmataeeshy, M.E.; Sokar, M.S.; Bahey-El-Din, M.; Shaker, D.S. Enhanced transdermal permeability of Terbinafine through novel nanoemulgel formulation; Development, in vitro and in vivo characterization. Future J. Pharm. Sci. 2018, 4, 18–28. [Google Scholar] [CrossRef]
- Meidan, V.M. Methods for quantifying intrafollicular drug delivery: A critical appraisal. Expert Opin. Drug Deliv. 2010, 7, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Richter, H.; Buettemeyer, R.; Huber, H.J.R.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. Differential stripping demonstrates a significant reduction of the hair follicle reservoir in vitro compared to in vivo. Eur. J. Pharm. Biopharm. 2008, 70, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Otberg, N.; Patzelt, A.; Rasulev, U.; Hagemeister, T.; Linscheid, M.; Sinkgraven, R.; Sterry, W.; Lademann, J. The role of hair follicles in the percutaneous absorption of caffeine. Br. J. Clin. Pharmacol. 2008, 65, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Patra, K.C.; Pareta, S.K.; Singh, J.; Rahman, M.A. Nanoemulsions as vehicles for transdermal delivery of glycyrrhizin. Braz. J. Pharm. Sci. 2011, 47, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Ramachandran, C.; Bielinska, A.U.; Kingzett, K.; Sun, R.; Weiner, N.D.; Roessler, B.J. Topical transfection using plasmid DNA in a water-in-oil nanoemulsion. Int. J. Pharm. 2001, 221, 23–34. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, J.; Baboota, S. Omega 3 fatty acid-enriched nanoemulsion of thiocolchicoside for transdermal delivery: Formulation, characterization and absorption studies. Drug Deliv. 2016, 23, 591–600. [Google Scholar] [CrossRef]
- Abd, E.; Benson, H.A.E.; Roberts, M.S.; Grice, J.E. Follicular Penetration of Caffeine from Topically Applied Nanoemulsion Formulations Containing Penetration Enhancers: In vitro Human Skin Studies. Skin Pharmacol. Physiol. 2018, 31, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jain, A.; Hurkat, P.; Jain, S.K. Transfollicular drug delivery: Current perspectives. Res. Rep. Transdermal Drug Deliv. 2016, 5, 1–17. [Google Scholar]
- Zhang, L.W.; Al-Suwayeh, S.A.; Hung, C.F.; Chen, C.C.; Fang, J.Y. Oil components modulate the skin delivery of 5-aminolevulinic acid and its ester prodrug from oil-in-water and water-in-oil nanoemulsions. Int. J. Nanomed. 2011, 6, 693–704. [Google Scholar] [Green Version]
- Syed Azhar, S.N.A.; Ashari, S.E.; Salim, N. Development of a kojic monooleate-enriched oil-in-water nanoemulsion as a potential carrier for hyperpigmentation treatment. Int. J. Nanomed. 2018, 13, 6465–6479. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Zhao, C.; Sun, D. Formation of nanoemulsion with long chain oil by W/O microemulsion dilution method. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 101–108. [Google Scholar] [CrossRef]
- Heuschkel, S.; Goebel, A.; Neubert, R.H.H. Microemulsions—Modern Colloidal Carrier for Dermal and Transdermal Drug Delivery. J. Pharm. Sci. 2008, 97, 603–631. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C.; Shapiro, L. New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug bioavailability in vivo. J. Control. Release 2004, 95, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Khachane, P.V.; Jain, A.S.; Dhawan, V.V.; Joshi, G.V.; Date, A.A.; Mulherkar, R.; Nagarsenker, M.S. Cationic nanoemulsions as potential carriers for intracellular delivery. Saudi Pharm. J. 2015, 23, 188–194. [Google Scholar] [CrossRef]
- Youenang Piemi, M.P.; Korner, D.; Benita, S.; Marty, J.P. Positively and negatively charged submicron emulsions for enhanced topical delivery of antifungal drugs. J. Control. Release 1999, 58, 177–187. [Google Scholar] [CrossRef]
- Liu, X.; Grice, J.E.; Lademann, J.; Otberg, N.; Trauer, S.; Patzelt, A.; Roberts, M.S. Hair follicles contribute significantly to penetration through human skin only at times soon after application as a solvent deposited solid in man. Br. J. Clin. Pharmacol. 2011, 72, 768–774. [Google Scholar] [CrossRef] [Green Version]
- Af-idah, B.M.; Nurahmanto, D.; Risky, D.D. Formulation and Optimization of Caffeine Nanoemulsion Using Factorial Design Study. In Proceedings of the ICMHS, Jember, Indonesia, 15–17 July 2017; pp. 6–9. [Google Scholar]
- Shaker, D.S.; Sloat, B.R.; Uyen, M.L.; Löhr, C.V.; Yanasarn, N.; Fischer, K.A.; Cui, Z. Immunization by application of DNA vaccine onto a skin area wherein the hair follicles have been induced into anagen-onset stage. Mol. Ther. 2007, 15, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech 2007, 8, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathan, I.B.; Mallikarjuna Setty, C. Nanoemulsion system for transdermal delivery of tamoxifen citrate: Design, Characterization, effect of penetration enhancers and in vivo studies. Dig. J. Nanomater. Biostruct. 2012, 7, 1373–1387. [Google Scholar]
- Sandig, A.G.; Campmany, A.C.C.; Campos, F.F.; Villena, M.J.M.; Naveros, B.C. Transdermal delivery of imipramine and doxepin from newly oil-in-water nanoemulsions for an analgesic and anti-allodynic activity: Development, characterization and in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Park, H.J. Stability investigation of hyaluronic acid based nanoemulsion and its potential as transdermal carrier. Carbohydr. Polym. 2011, 83, 1303–1310. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, V.K.; Singh, O.P.; Shafaat, K.; Kumar, S.; Ahmad, F.J. Formulation and optimization of nanoemulsion using antifungal lipid and surfactant for accentuated topical delivery of Amphotericin B. Drug Deliv. 2016, 23, 3101–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratap, S.B.; Brajesh, K.; Jain, S.K.; Kausar, S. Development and Characterization of A Nanoemulsion Gel formulation for Transdermal delivery of Carvedilol for Transdermal delivery of Carvedilol. Int. J. Drug Dev. Res. 2012, 4, 151–161. [Google Scholar]
- Zaid Alkilani, A.; Hamed, R.; Al-Marabeh, S.; Kamal, A.; Abu-Huwaij, R.; Hamad, I. Nanoemulsion-based film formulation for transdermal delivery of carvedilol. J. Drug Deliv. Sci. Technol. 2018, 46, 122–128. [Google Scholar] [CrossRef]
- Lou, H.; Qiu, N.; Crill, C.; Helms, R.; Almoazen, H. Development of W/O Microemulsion for Transdermal Delivery of Iodide Ions. AAPS PharmSciTech 2013, 14, 168–176. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Souto, E.B.; Boonme, P.; Müller, R.H. Q10-loaded NLC versus nanoemulsions: Stability, rheology and in vitro skin permeation. Int. J. Pharm. 2009, 377, 207–214. [Google Scholar] [CrossRef]
- Baboota, S.; Shakeel, F.; Ahuja, A.; Ali, J.; Shafiq, S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007, 57, 315–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aqil, M.; Kamran, M.; Ahad, A.; Imam, S.S. Development of clove oil based nanoemulsion of olmesartan for transdermal delivery: Box–Behnken design optimization and pharmacokinetic evaluation. J. Mol. Liq. 2016, 214, 238–248. [Google Scholar] [CrossRef]
- Arora, R.; Aggarwal, G.; Harikumar, S.L.; Kaur, K. Nanoemulsion Based Hydrogel for Enhanced Transdermal Delivery of Ketoprofen. Adv. Pharm. 2014, 2014, 468456. [Google Scholar] [CrossRef]
- Wais, M.; Samad, A.; Nazish, I.; Khale, A.; Aqil, M.; Khan, M. Formulation Development Ex-Vivo and in-Vivo Evaluation of Nanoemulsion for transdermal delivery of glibenclamide. Int. J. Pharm. Pharm. Sci. 2013, 5, 747–754. [Google Scholar]
- Abd, E.; Namjoshi, S.; Mohammed, Y.H.; Roberts, M.S.; Grice, J.E. Synergistic Skin Penetration Enhancer and Nanoemulsion Formulations Promote the Human Epidermal Permeation of Caffeine and Naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ko, J.A.; Kim, J.T.; Cha, D.S.; Cho, J.H.; Park, H.J.; Shin, G.H. Preparation of a Capsaicin-Loaded Nanoemulsion for Improving Skin Penetration. J. Agric. Food Chem. 2014, 62, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, J.; Zheng, H.; Zhang, R.; Han, Y. The preparation of 3,5-dihydroxy-4-isopropylstilbene nanoemulsion and in vitro release. Int. J. Nanomed. 2011, 6, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, G.; Dhawan, B.; Harikumar, S. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int. J. Pharm. Investig. 2014, 4, 65. [Google Scholar] [CrossRef]
- Abolmaali, S.S.; Tamaddon, A.M.; Farvadi, F.S.; Daneshamuz, S.; Moghimi, H. Pharmaceutical nanoemulsions and their potential topical and transdermal applications. Iran. J. Pharm. Sci. 2011, 7, 139–150. [Google Scholar]
- Ki, H.; Choi, H. The Effect of Meloxicam/Ethanolamine Salt Formation on Per-cutaneous Absorption of Meloxicam. Arch. Pharm. Res. 2007, 30, 215–221. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K.; Singh, J. Lipid extraction and transport of hydrophilic solutes through porcine epidermis. Int. J. Pharm. 2001, 225, 75–82. [Google Scholar] [CrossRef]
- Mostafa, D.M.; El-Alim, S.H.A.; Asfour, M.H.; Al-Okbi, S.Y.; Mohamed, D.A.; Awad, G. Transdermal nanoemulsions of Foeniculum vulgare Mill. essential oil: Preparation, characterization and evaluation of antidiabetic potential. J. Drug Deliv. Sci. Technol. 2015, 29, 99–106. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.W.; Huang, M.T.; Ho, C.T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.S.; Weisspapir, M.R.; Friedman, D.I. Enhanced transdermal delivery of diazepam by submicron emulsion (SME) creams. Pharm. Res. 1995, 12, 687–692. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.M.; Mostafa, D.M.; Ghoneim, A.M. Self-nanoemulsifying drug delivery system for sertraline hydrochloride: Design, Preparation and characterization. Int. J. Pharm. Pharm. Sci. 2014, 6, 589–595. [Google Scholar]
- Di Federico, V.; Longo, S.; King, S.E.; Chiapponi, L.; Petrolo, D.; Ciriello, V. Gravity-driven flow of Herschel-Bulkley fluid in a fracture and in a 2D porous medium. J. Fluid Mech. 2017, 821, 59–84. [Google Scholar] [CrossRef]
- Baspinar, Y.; Borchert, H.H. Penetration and release studies of positively and negatively charged nanoemulsions—Is there a benefit of the positive charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef]
- Wang, J.-J.; Sung, K.C.; Hu, O.Y.-P.; Yeh, C.-H.; Fang, J.-Y. Submicron lipid emulsion as a drug delivery system for nalbuphine and its prodrugs. J. Control. Release 2006, 115, 140–149. [Google Scholar] [CrossRef]
- Su, R.; Yang, L.; Wang, Y.; Yu, S.; Guo, Y.; Deng, J.; Zhao, Q.; Jin, X. Formulation, development, and optimization of a novel octyldodecanol-based nanoemulsion for transdermal delivery of ceramide IIIB. Int. J. Nanomed. 2017, 12, 5203–5221. [Google Scholar] [CrossRef]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Peira, E.; Carlotti, M.E.; Trotta, C.; Cavalli, R.; Trotta, M. Positively charged microemulsions for topical application. Int. J. Pharm. 2008, 346, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Coutinho, C.; Santos-Oliveira, R.; dos Santos, E.; Mansur, C.R. Development of a photoprotective and antioxidant nanoemulsion containing chitosan as an agent for improving skin retention. Eng. Life Sci. 2015, 15, 593–604. [Google Scholar] [CrossRef]
- Yilmaz, E.; Borchert, H.-H. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema—An in vivo study. Int. J. Pharm. 2006, 307, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Borchert, H.H. Design of a phytosphingosine-containing, positively-charged nanoemulsion as a colloidal carrier system for dermal application of ceramides. Eur. J. Pharm. Biopharm. 2005, 60, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ouyang, W.-Q.; Wei, Y.-P.; Syed, S.; Hao, C.-S.; Wang, B.-Z.; Shang, Y.-H. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int. J. Nanomed. 2016, 11, 5971–5987. [Google Scholar] [CrossRef] [PubMed]
- Malgope, A.; Murthy, P.N.; Ramani, R.; Dey, S. Development of Nanoemulsion as Carrier for Transdermal Delivery of Valsartan. Int. J. Pharm. Chem. Sci. 2013, 2, 1655–1665. [Google Scholar]
- Lucca, L.G.; de Matos, S.P.; Borille, B.T.; Dias, D.D.; Teixeira, H.F.; Veiga, V.F., Jr.; Limberger, R.P.; Koester, L.S. Determination of β-caryophyllene skin permeation/retention from crude copaiba oil (Copaifera multijuga Hayne) and respective oil-based nanoemulsion using a novel HS-GC/MS method. J. Pharm. Biomed. Anal. 2015, 104, 144–148. [Google Scholar] [CrossRef]
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm. 2018, 545, 197–205. [Google Scholar] [CrossRef]
- Baroli, B.; López-Quintela, M.A.; Delgado-Charro, M.B.; Fadda, A.M.; Blanco-Méndez, J. Microemulsions for topical delivery of 8-methoxsalen. J. Control. Release 2000, 69, 209–218. [Google Scholar] [CrossRef]
- Warner, R.R.; Stone, K.J.; Boissy, Y.L. Hydration Disrupts Human Stratum Corneum Ultrastructure. J. Investig. Dermatol. 2003, 120, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M.; Holleran, W.M.; Feingold, K.R.; Tsai, J.; Menon, G.K. The Potential of Metabolic Interventions to Enhance Transdermal Drug Delivery. J. Investig. Dermatol. Symp. Proc. 2002, 7, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hal, D.A.; Jeremiasse, E.; Junginger, H.E.; Spies, F.; Bouwstra, J.A. Structure of fully hydrated human stratum corneum: A freeze-fracture electron microscopy study. J. Investig. Dermatol. 1996, 106, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Kemken, J.; Ziegler, A.; Müller, B.W. Influence of supersaturation on the pharmacodynamic effect of bupranolol after dermal administration using microemulsions as vehicle. Pharm. Res. 1992, 9, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Topgaard, D.; Kocherbitov, V.; Sousa, J.J.S.; Pais, A.A.C.C.; Sparr, E. Stratum corneum hydration: Phase transformations and mobility in stratum corneum, extracted lipids and isolated corneocytes. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2647–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, L. Overcoming the Cutaneous Barrier with Microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.R.; Jain, S.K.; Varshney, M. AOT water-in-oil microemulsions as a penetration enhancer in transdermal drug delivery of 5-fluorouracil. Colloids Surf. B Biointerfaces 2005, 41, 25–32. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Geneidi, A.S.; Guy, R.H. Uptake of Microemulsion Components into the Stratum Corneum and Their Molecular Effects on Skin Barrier Function. Mol. Pharm. 2010, 7, 1266–1273. [Google Scholar] [CrossRef]
- Mathur, V.; Satrawala, Y.; Rajput, M.S. Physical and chemical penetration enhancers in transdermal drug delivery system. Asian J. Pharm. 2014, 4. [Google Scholar] [CrossRef]
- Bhatia, G.; Zhou, Y.; Banga, A.K. Adapalene Microemulsion for Transfollicular Drug Delivery. J. Pharm. Sci. 2013, 102, 2622–2631. [Google Scholar] [CrossRef] [PubMed]



| Tests | Equipment | Significance | Description | Ref |
|---|---|---|---|---|
| Visual inspection | Naked eye | To determine the successful formation of the NE. | Visual observation of sudden turbidity followed by the production of clear and transparent NE. | [103] |
| Viscosity | Rotational viscometer | Low viscosity NE has faster release and rapid skin penetration than high viscosity NE, o/w NE are usually low in viscosity compared to the w/o NE. | Measurement of the amount of torque needed to rotate the paddle in the NE. | [104,105,106] |
| Morphology | Transmission electron microscopy (TEM) Scanning electron microscope (SEM) Atomic force microscopy (AFM) Neutron scattering Ultrasonic resonator technology Cryo-electron microscope | To verify the droplets fabricated with enough consistency in their shape and size being in the nano-range. | The NE sample is negatively stained with a 1% solution of phosphotungstic acid and then applied to the copper or carbon coated grid, depending on the model of the TEM or SEM used. The accelerating voltage used is usually 20 kV, and by using the appropriate software and magnification, a quantitative measurement can be achieved, along with the quality and consistency of the NE drops. | [107,108,109] |
| Particle size Polydispersity index (PDI) Zeta potential (ZP) | Photon correlation spectroscopy (PCS) Dynamic light scattering (DLS) | To quantify the homogeneity and dispersion of the NE as well as to estimate the broadness and range of the droplet size. The lower the PDI value (<0.2) and the higher the ZP, the better NE stability is against onward ripping and other destabilizing forces. | Size and size distribution are measured from the collected measurements of the scattering of dynamic light of the NE droplets. ZP is measured as the potential charge difference between the particles and the continuous phase. | [110,111,112] |
| Electro-conductivity | A conductivity meter | An indication of preliminary change in NE droplet size change although the relationship between electrical conductivity and NE instability is not linear. | The meter measures the amount of electrical current or conductance in the NE sample. The meter, equipped with a probe, is placed into the sample to be measured. The meter applies a voltage between two electrodes inside the probe. Electrical resistance from the total dispersed particles in sample causes a drop in voltage, which is read by the meter. | [113,114] |
| Refractive index | A refractometer | A strong indication of uniformity and the formation of an isotropic NE. | Comparing the refractive index (RI) of the NE with water (RI = 1.333), where the closer the NE value to the water value, the more uniform and transparent the NE is. | [115,116] |
| In vitro skin permeation | Franz diffusion cell apparatus | To assess transcutaneous penetration or membrane retention. | A sample of NE is placed into the donor compartment after placing a variety of membranes, either synthetic or excised skin from an animal model. The receiver compartment of the Franz diffusion cell is filled with a phosphate buffer saline with a pH of 7.4, which simulates the blood stream. It is then stirred at 100 rpm at a temperature of 37 °C. A sample of 1 mL is taken either manually or automatically, filtered, then analyzed using UV spectroscopy or HPLC. Once the value of the released drug has been determined for every hour, the steady state flux (Jss) is calculated with the formula Jss = P.CD, where P is the permeability coefficient and CD is the donor chamber concentration. | [19,117] |
| In vivo dermato-pharmacokinetics and pharmacodynamics | Intact live animals HPLC | To establish a plasma drug concentration-time profile or to assess a pharmacological drug effect. | Administering NE to a shaved animal skin. Blood samples are withdrawn at different intervals, centrifuged, and then the plasma is analyzed using HPLC to determine the amount of drug that reached circulation. Furthermore, the pharmacodynamics properties of the NE are assessed depending on the pharmacological effect of the drug. | [36,84,118,119,120] |
| Skin irritation | Live animals (rats or rabbits) | To determine whether NE produced irritation or not. | The healthy experimental animals were divided into groups and the formulation was applied on the hair-free skin of animals by uniform spreading within a specific area. The experiment was usually carried out for 7 days and the application sites were graded according to a visual scoring scale. The sites tested were put under observation for 48 h to detect if any erythema or edema was formed after application. Skin irritation was scored following the Draize method. | [121,122,123,124] |
| Permeant | Nanoemulsion Components | Underlying Mechanism of Penetration | Ref. | |||
|---|---|---|---|---|---|---|
| Oil Phase | Surfactant | Co-Surfactant | Aqueous Phase | |||
| Ropinirole hydrochloride | Brij 30 | Brij 35 + Brij 30 | Isopropyl alcohol (IPA) | Water | Enhanced transdermal delivery is mainly attributed to the thermodynamic activity of the drug along with the low viscosity, resulting in a shortened release lag time from 12 to 2.7 h. Increasing the ethanol content from 20 to 30% showed a slight increase in flux from 20.25 to 25.94 µg/cm2. | [127] |
| Inulin | Olive oil | Tween 80 | No co-surfactant used | Water | Using a low hydrophile-lipophile balance (HLB) surfactant mixture showed better penetration compared to aqueous and other micellar formulations. This enhancement in follicular penetration is attributed to the solubilization of sebum by NE components. | [130] |
| Glycyrrhizin | Soybean oil | Span 80 | Brij 35 + IPA | Water | The selected optimum formula showed enhanced and sustained release profile on human skin. Results are attributed to low droplet size and viscosity. | [136] |
| DNA plasmid | Olive oil | Tween 80 | No co-surfactant used | Water | When DNA plasmid was loaded into w/o NE, a more condensed state of DNA was formed, resulting in higher gene expression levels due to its deposition in hair follicles. | [137] |
| Thiocolchicoside | Linseed oil | Span 80 | Transcutol P | Water | A 5-fold increase in the penetration of thiocolchicoside (TCC) was reported. The small droplet size as well as the NE components acting as penetration enhancers are the main driving factors for transdermal enhancement. | [138] |
| Caffeine | OA/EU | Volpo-N10 | Ethanol | Water | Caffeine loaded NE formulations showed the transport of 51% and 54% of the drug, compared to 27% in case of a topical caffeine solution. This is attributed to the transfollicular route by NE. | [139] |
| 5-Aminolevulinic acid | Soybean oil | Span 80 | α-terpineol | Water | The o/w 5-aminolevulinic acid (ALA) showed a high flux rate, but not the w/o NE, which is mostly attributed to NE components. The addition of α-terpineol, which is a penetration enhancer, did not yield any further improvement in penetration. The thermodynamic activity of the drug is also one of the contributing factors. | [141] |
| Permeant | NE Components | Underlying Mechanism of Penetration | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Oil Phase | Surfactant | Co-Surfactant | PE | Aq. Phase | |||
| Aceclofenac | Labrafil | Tween 80 | Transcutol P | ---- | Water | Compared to aceclofenac gel, the developed NE formulation showed an increased anti-inflammatory effect and better penetration. The suggested mechanism is the low droplet size and viscosity. | [151] |
| Celecoxib | Sefsol-218 and Triacetin | Cremophor-EL/Tween 80 | Transcutol P | ---- | Water | The optimized formulation showed the highest permeation value, with an inhibition of 70.8% of inflammation area. The permeation efficiency of celecoxib is attributed to the small droplet size, low viscosity, and permeation enhancement factor of both the hydrophilic and hydrophobic domains in the NE phases. | [160] |
| Olmesartan | Clove oil | Tween 20 | PEG | --- | Water | The NE formulation exhibited a prolonged Tmax with a large AUC value giving the olmesartan NE great bioavailability compared to its oral counterpart. The small droplet size and NE components enhancing skin penetration were the underlying mechanisms here. | [161] |
| Ketoprofen | OA | Tween 80 | Transcutol P | --- | Water | The NE formulation showed better permeation results in comparison with the plain drug gel, drug solution, and the marketed formulation, Transcutol P. The permeation enhancing ability played a major role. Incorporating the NE into a gel showed no improvement in flux, indicating the NE components, along with the small droplet size, is what led to such a profound skin permeation ability. | [162] |
| Glibenclamide | Labrafac and tricetin | Tween 80 | Diethylene glycol monoethyl ether | --- | Water | The bioavailability of GLBD was enhanced by 3.92 times compared to the oral formulation. The NE small droplet size and penetration enhancement ability of NE components are the underlying mechanisms. | [163] |
| Clozapine | OA | Tween 20 | Transcutol P | --- | Water | The combination of OA along with Tween 20 and Transcutol P showed great penetration enhancing ability, allowing a 3-fold improvement in transdermal release compared to the traditional emulsion. Here, the enhancing ability of the NE components is the mechanism that successfully aided the delivery of the drug transdermally. | [10] |
| Tamoxifen | OA | Chromophore RH40 | Ethanol | Dill essential oil | Water | Good penetration results were reported with the selected formulation which had the smallest droplet size, and the addition of 5% Dill essential oil showed a profound effect on tamoxifen flux. | [152] |
| Caffeine and Naproxen | OA and EU | Volpo N10 | Ethanol | ---- | Water | Using human skin in this study, it was demonstrated that both caffeine and naproxen showed enhanced human epidermal permeation, mostly through the solubilizing of the active elements in the NE components, followed by NE components ability to disrupt the lipid bilayer of the SC cells. | [164] |
| Cumin | OA | Tween 20 | Ethanol | ---- | Water | The plasma total antioxidant capacity reached maximum efficiency after 7 days of transdermal application of cumin NE. The enhancement ability of these three components in the NE played a big role in raising the antioxidant activity systemically using cumin. | [5] |
| Imipramine and doxepin | OA | Labrasol | Plural oleique | Limonene | Water | For both drugs, the resulted NE droplet size was below 28 nm. The selection of OA as an oil phase was due to its ability to fluidize the lipid bilayer of the SC, along with 5% terpene (limonene). This gave promising results, showing both the local and systemic analgesic activity of the drugs. Low droplet size and disruption of SC lipid bilayer was the underlying mechanism of penetration. | [153] |
| Vitamin E | Methylene chloride | Tween 80 | ---- | Water | Droplet size reduction and positive charge NE binding to the skin are important influencers in the successful transdermal delivery of vitamin E from hyaluronic acid-based NE. | [154] | |
| Capsaicin | Oleoresin | Tween 80 and Span 80 | --- | --- | Water | Using a CLSM to determine the depth of penetration for a capsaicin loaded NE, fluorescence intensity was detected through all skin layers, indicating the successful penetration of the NE formulation. Droplet sizes of 20 to 62 nm and the optimization of the HLB value of Smix were the main contributors to such successful results. | [165] |
| Amphotericin B | CPG8 | LAB | PEG 400 | --- | Water | Antifungal NE was developed and compared to formulation in the market Amphotericin B NE showed greater penetration results than aqueous dye and commercial product using CLSM. Results are attributed to NE components ability to enhance transdermal delivery through SC. | [155] |
| Carvedilol | OA and IPM | Tween20 | Carbitol | --- | Water | An improvement of 1.72-fold in the AUC was reported with the carvedilol NE over the oral formulation. Such results were mainly attributed to the optimum droplet size and low viscosity. | [156,157] |
| Meloxicam | Caprylic acid | Tween 80 | PEG 400 | --- | Water | The synergetic properties of the NE components contributed towards the enhanced penetration of MLX into the skin, altering the tightly packed nature of the SC. | [111] |
| 3,5-Dihydroxy-4-isopropylstilbene | IPM | Cremophor EL 40 | Ethanol, n-butanol, n-propanol, 1,2propanediol | --- | Water | The usage of four types of co-surfactants and oil droplet nano-sizing were what led to good in vitro drug release results. | [166] |
| Piroxicam | OA | Tween 80 | Ethanol | --- | Water | A piroxicam NE was developed and incorporated into an emulgel. Even though incorporating the NE into a gel form eased applicability to the skin, flux was noticeably reduced compared to normal the NE. By using 35% ethanol, low viscosity of the formulation was the leading mechanism here. | [167] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm87030017
Shaker DS, Ishak RAH, Ghoneim A, Elhuoni MA. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Scientia Pharmaceutica. 2019; 87(3):17. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm87030017
Chicago/Turabian StyleShaker, Dalia S., Rania A. H. Ishak, Amira Ghoneim, and Muaeid A. Elhuoni. 2019. "Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs" Scientia Pharmaceutica 87, no. 3: 17. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm87030017