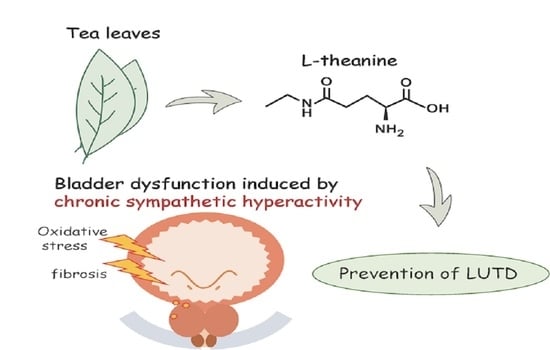
l-Theanine Protects Bladder Function by Suppressing Chronic Sympathetic Hyperactivity in Spontaneously Hypertensive Rat
Abstract
:1. Introduction
2. Results
2.1. Voiding Behaviors in SHRs after 6 Weeks
2.2. Cystometric Parameters in Conscious Rats
2.3. Assessment of Bladder Contractile Strength
2.4. Expression of Oxidative Stress Markers in the Bladder
2.5. Histological Changes in the Bladder Muscle Layer
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Metabolic Cage Experiments
4.3. Conscious Free-Moving Cystometry
4.4. Organ Bath Study
4.5. Detection of Oxidative Stress Markers
4.6. Histological Examination
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baladia, E.; Basulto, J.; Manera, M.; Martínez, R.; Calbet, D. Effect of green tea or green tea extract consumption on body weight and body composition; systematic review and meta-analysis. Nutr. Hosp. 2014, 29, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jang, Y.H.; Kim, Y.S.; Kim, J.; Seong, B.L. Evaluation of green tea extract as a safe personal hygiene against viral infections. J. Biol. Eng. 2018, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Durgo, K.; Kostić, S.; Gradiški, K.; Komes, D.; Osmak, M.; Franekić, J. Genotoxic effects of green tea extract on human laryngeal carcinoma cells in vitro. Arh. Hig. Rada Toksikol. 2011, 62, 139–146. [Google Scholar] [CrossRef]
- Sing, M.F.; Yang, W.S.; Gao, S.; Gao, J.; Xiang, Y.B. Epidemiological studies of the association between tea drinking and primary liver cancer: A meta-analysis. Eur. J. Cancer Prev. 2011, 20, 157–165. [Google Scholar] [CrossRef]
- Terashima, T.; Takido, J.; Yokogoshi, H. Time-dependent changes of amino acids in the serum, liver, brain and urine of rats administered with theanine. Biosci. Biotechnol. Biochem. 1999, 63, 615–618. [Google Scholar] [CrossRef]
- Yoto, A.; Motoki, M.; Murao, S.; Yokogoshi, H. Effects of l-theanine or caffeine intake on changes in blood pressure under physical and psychological stresses. J. Physiol. Anthropol. 2012, 31, 28. [Google Scholar] [CrossRef] [Green Version]
- Nobre, A.C.; Rao, A.; Owen, G.N. l-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 167–168. [Google Scholar]
- Haskell, C.F.; Kennedy, D.O.; Milne, A.L.; Wesnes, K.A.; Scholey, A.B. The effects of l-theanine, caffeine and their combination on cognition and mood. Biol. Psychol. 2008, 77, 113–122. [Google Scholar] [CrossRef]
- Ogawa, S.; Ota, M.; Ogura, J.; Kato, K.; Kunugi, H. Effects of l-theanine on anxiety-like behavior, cerebrospinal fluid amino acid profile, and hippocampal activity in Wistar Kyoto rats. Psychopharmacology 2018, 235, 37–45. [Google Scholar] [CrossRef]
- Unno, K.; Tanida, N.; Ishii, N.; Yamamoto, H.; Iguchi, K.; Hoshino, M.; Takeda, A.; Ozawa, H.; Ohkubo, T.; Juneja, L.R.; et al. Anti-stress effect of theanine on students during pharmacy practice: Positive correlation among salivary α-amylase activity, trait anxiety and subjective stress. Pharmacol. Biochem. Behav. 2013, 111, 128–135. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, A.; Roy, S.; Bera, B.; Bandyopadhyay, S.K. l-Theanine healed NSAID-induced gastric ulcer by modulating pro/antioxidant balance in gastric ulcer margin. J. Nat. Med. 2014, 68, 699–708. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Nomiya, M.; Andersson, K.E. Functional consequences of chronic bladder ischemia. Neurourol. Urodyn. 2014, 33, 54–58. [Google Scholar] [CrossRef]
- Wróbel, A.; Serefko, A.; Woźniak, A.; Kociszewski, J.; Szopa, A.; Wiśniewski, R.; Poleszak, E. Duloxetine reverses the symptoms of overactive bladder co-existing with depression via the central pathways. Pharmacol. Biochem. Behav. 2020, 189, 172842. [Google Scholar] [CrossRef]
- Keske, M.; Gok, B.; Ener, K.; Ozcan, M.F.; Ozayar, A.; Okulu, E.; Neselioglu, S.; Cakmak, S.; Asil, E.; Aldemir, M.; et al. Relationship between oxidative stress and detrussor overactivity: A case control study. Urol. J. 2019, 16, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, H.; Cengiz, H. Association between Metabolic Syndrome and Serum Nerve Growth Factor Levels in Women with Overactive Bladder. Gynecol. Obstet. Investig. 2018, 83, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Spitsbergen, J.M.; Clemow, D.B.; McCarty, R.; Steers, W.D.; Tuttle, J.B. Neurally mediated hyperactive voiding in spontaneously hypertensive rats. Brain Res. 1998, 790, 151–159. [Google Scholar] [CrossRef]
- Saito, M.; Ohmasa, F.; Tsounapi, P.; Inoue, S.; Dimitriadis, F.; Kinoshita, Y.; Satoh, K. Nicorandil ameliorates hypertension-related bladder dysfunction in the rat. Neurourol. Urodyn. 2012, 31, 695–701. [Google Scholar] [CrossRef]
- Persson, K.; Pandita, R.K.; Spitsbergen, J.M.; Steers, W.D.; Tuttle, J.B.; Andersson, K.E. Spinal and peripheral mechanisms contributing to hyperactive voiding in spontaneously hypertensive rats. Am. J. Physiol. 1998, 275, R1366–R1373. [Google Scholar] [CrossRef]
- Minami, M.; Togashi, H.; Kurosawa, M.; Shimamura, K.; Koike, Y.; Saito, H.; Kobayashi, T.; Yasuda, H. Changes in circadian rhythms of urinary catecholamine excretion in cold-stressed spontaneously hypertensive rats. Hokkaido Igaku Zasshi 1982, 57, 55–56. [Google Scholar]
- Yokogoshi, H.; Kato, Y.; Sagesaka, Y.M.; Takihara-Matsuura, T.; Kakuda, T.; Takeuchi, N. Reduction effect of theanine on blood pressure and brain 5-hydroxyindoles in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 1995, 59, 615–618. [Google Scholar] [CrossRef] [Green Version]
- Szemeredi, K.; Bagdy, G.; Stull, R.; Keiser, H.R.; Kopin, I.J.; Goldstein, D.S. Sympathoadrenomedullary hyper-responsiveness to yohimbine in juvenile spontaneously hypertensive rats. Life Sci. 1988, 43, 1063–1068. [Google Scholar] [CrossRef]
- Safar, M.E.; Lacolley, P. Disturbance of macro- and microcirculation: Relations with pulse pressure and cardiac organ damage. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1–H7. [Google Scholar] [CrossRef] [PubMed]
- Yannoutsos, A.; Levy, B.I.; Safar, M.E.; Slama, G.; Blacher, J. Pathophysiology of hypertension: Interactions between macro and microvascular alterations through endothelial dysfunction. J. Hypertens. 2014, 32, 216–224. [Google Scholar] [CrossRef]
- Akaihata, H.; Hata, J.; Tanji, R.; Honda-Takinami, R.; Matsuoka, K.; Sato, Y.; Kataoka, M.; Ogawa, S.; Kojima, Y. Tetrahydrobiopterin prevents chronic ischemia-related lower urinary tract dysfunction through the maintenance of nitric oxide bioavailability. Sci. Rep. 2020, 10, 19844. [Google Scholar] [CrossRef]
- Shimizu, S.; Saito, M.; Oiwa, H.; Ohmasa, F.; Tsounapi, P.; Oikawa, R.; Dimitriadis, F.; Martin, D.T.; Satoh, I.; Kinoshita, Y.; et al. Olmesartan ameliorates urinary dysfunction in the spontaneously hypertensive rat via recovering bladder blood flow and decreasing oxidative stress. Neurourol. Urodyn. 2014, 33, 350–357. [Google Scholar] [CrossRef]
- Nagao, Y.; Shimizu, S.; Kurabayashi, A.; Shimizu, T.; Tsuda, M.; Higashi, Y.; Fujieda, M.; Saito, M. Effects of silodosin and tadalafil on bladder dysfunction in spontaneously hypertensive rats: Possible role of bladder blood flow. Int. J. Urol. 2020, 27, 258–265. [Google Scholar] [CrossRef]
- Birder, L.A.; Ruggieri, M.; Takeda, M.; van Koeveringe, G.; Veltkamp, S.; Korstanje, C.; Parsons, B.; Fry, C.H. How does the urothelium affect bladder function in health and disease? ICI-RS 2011. Neurourol. Urodyn. 2012, 31, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akaihata, H.; Nomiya, M.; Matsuoka, K.; Koguchi, T.; Hata, J.; Haga, N.; Kushida, N.; Ishibashi, K.; Aikawa, K.; Kojima, Y. Protective Effect of a Rho-kinase Inhibitor on Bladder Dysfunction in a Rat Model of Chronic Bladder Ischemia. Urology 2018, 111, 238.e7–238.e12. [Google Scholar] [CrossRef]
- Zhang-James, Y.; Middleton, F.A.; Faraone, S.V. Genetic architecture of Wistar-Kyoto rat and spontaneously hypertensive rat substrains from different sources. Physiol. Genom. 2013, 45, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.L.; Ely, D.L.; Turner, M.E. Genetic divergence between the Wistar-Kyoto rat and the spontaneously hypertensive rat. Hypertension 1992, 19, 425–427. [Google Scholar] [CrossRef] [Green Version]
- Mahjani, B.; Koskela, L.R.; Mahjani, C.G.; Janecka, M.; Batuure, A.; Hultman, C.M.; Reichenberg, A.; Buxbaum, J.D.; Akre, O.; Grice, D.E. Systematic review and meta-analysis: Relationships between attention-deficit/hyperactivity disorder and urinary symptoms in children. Eur. Child Adolesc. Psychiatry 2021, 1–8. [Google Scholar] [CrossRef]
- Willette, R.N.; Sauermelch, C.F.; Hieble, J.P. Role of alpha-1 and alpha-2 adrenoceptors in the sympathetic control of the proximal urethra. J. Pharmacol. Exp. Ther. 1990, 252, 706–710. [Google Scholar] [PubMed]
- Saito, M.; Shimizu, S.; Ohmasa, F.; Oikawa, R.; Tsounapi, P.; Dimitriadis, F.; Kinoshita, Y.; Satoh, K. Characterization of silodosin and naftopidil in the treatment of bladder dysfunction in the spontaneously hypertensive rat. Neurourol. Urodyn. 2013, 32, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, M.; Burmeister, D.M.; Sawada, N.; Campeau, L.; Zarifpour, M.; Keys, T.; Peyton, C.; Yamaguchi, O.; Andersson, K.E. Prophylactic effect of tadalafil on bladder function in a rat model of chronic bladder ischemia. J. Urol. 2013, 189, 754–761. [Google Scholar] [CrossRef]
- Rosei, E.A.; Rizzoni, D.; Castellano, M.; Porteri, E.; Zulli, R.; Muiesan, M.L.; Bettoni, G.; Salvetti, M.; Muiesan, P.; Giulini, S.M. Media: Lumen ratio in human small resistance arteries is related to forearm minimal vascular resistance. J. Hypertens. 1995, 13, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P. The structural factor of hypertension: Large and small artery alterations. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef]








| SHR-Water | SHR-Theanine | p Value | |||||
|---|---|---|---|---|---|---|---|
| Fluid intake (mL/day) | 33.9 | ± | 2.8 | 33.8 | ± | 1.7 | 1.000 |
| Body weight (g) | 353.0 | ± | 12.3 | 354.0 | ± | 11.3 | 0.912 |
| Bladder weight (g) | 0.19 | ± | 0.03 | 0.22 | ± | 0.04 | 0.515 |
| HR (beats/min) | 412.3 | ± | 18.5 | 391.9 | ± | 20.7 | 0.606 |
| SBP (mmHg) | 204.0 | ± | 5.8 | 188.8 | ± | 3.8 | 0.074 |
| MBP (mmHg) | 173.3 | ± | 4.3 | 159.9 | ± | 3.3 | 0.046 * |
| DBP (mmHg) | 159.3 | ± | 3.9 | 147.9 | ± | 4.1 | 0.074 |
| SHR-Water | SHR-Theanine | p Value | |||||
|---|---|---|---|---|---|---|---|
| Urine volume (g/24-h) | 14.4 | ± | 3.3 | 12.9 | ± | 3.2 | 0.393 |
| Micturition frequency (count/24-h) | 22.3 | ± | 5.3 | 16.3 | ± | 4.0 | 0.004 * |
| Daytime frequency (count/12-h) | 5.0 | ± | 1.6 | 5.1 | ± | 1.2 | 0.796 |
| Nighttime frequency (count/12-h) | 16.3 | ± | 4.3 | 11.2 | ± | 3.6 | 0.007 * |
| Single voided volume (g) | 0.67 | ± | 0.19 | 0.81 | ± | 0.15 | 0.043 * |
| SHR-Water | SHR-Theanine | p Value | |||||
|---|---|---|---|---|---|---|---|
| Intercontractile interval (min) | 5.6 | ± | 8.4 | 7.8 | ± | 1.7 | 0.002 * |
| Baseline pressure (cmH2O) | 9.59 | ± | 5.56 | 8.93 | ± | 4.83 | 0.739 |
| Voided volume (g) | 0.96 | ± | 0.18 | 1.34 | ± | 0.35 | 0.029 * |
| Residual urine volume (mL) | 0.16 | ± | 0.08 | 0.22 | ± | 0.13 | 0.247 |
| Maximum pressure (cmH2O) | 47.1 | ± | 16.9 | 70.4 | ± | 33.1 | 0.123 |
| Bladder compliance (g/cmH2O) | 0.12 | ± | 0.79 | 0.11 | ± | 0.03 | 0.393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, K.; Akaihata, H.; Hata, J.; Tanji, R.; Honda-Takinami, R.; Onagi, A.; Hoshi, S.; Koguchi, T.; Sato, Y.; Kataoka, M.; et al. l-Theanine Protects Bladder Function by Suppressing Chronic Sympathetic Hyperactivity in Spontaneously Hypertensive Rat. Metabolites 2021, 11, 778. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110778
Matsuoka K, Akaihata H, Hata J, Tanji R, Honda-Takinami R, Onagi A, Hoshi S, Koguchi T, Sato Y, Kataoka M, et al. l-Theanine Protects Bladder Function by Suppressing Chronic Sympathetic Hyperactivity in Spontaneously Hypertensive Rat. Metabolites. 2021; 11(11):778. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110778
Chicago/Turabian StyleMatsuoka, Kanako, Hidenori Akaihata, Junya Hata, Ryo Tanji, Ruriko Honda-Takinami, Akifumi Onagi, Seiji Hoshi, Tomoyuki Koguchi, Yuichi Sato, Masao Kataoka, and et al. 2021. "l-Theanine Protects Bladder Function by Suppressing Chronic Sympathetic Hyperactivity in Spontaneously Hypertensive Rat" Metabolites 11, no. 11: 778. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110778