Impacts of Constitutive and Induced Benzoxazinoids Levels on Wheat Resistance to the Grain Aphid (Sitobion avenae)
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Wheat Resistance to S. avenae
2.2. Chromatographic and Mass Spectrometric Behavior of Benzoxazinoids
2.3. Constitutive Benzoxazinoid Levels in Wheat Seedlings and Their Correlation with S. avenae Resistance
2.4. Induced Levels of Benzoxazinoids in Aphid Infested Wheat Seedlings and Their Correlation with S. avenae Resistance
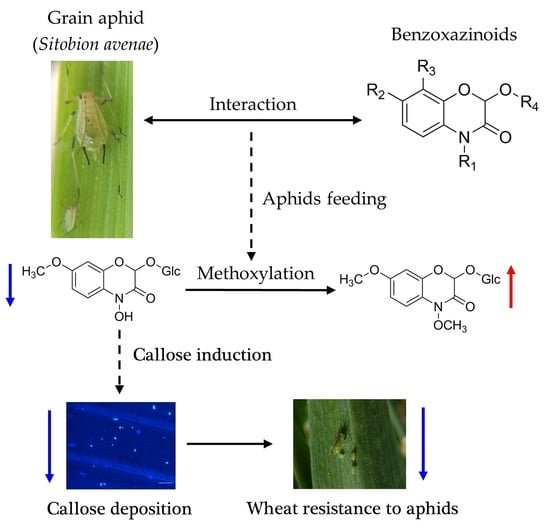
2.5. The Relationship between Aphid-Induced Benzoxazinoids Levels and Callose Deposition
3. Discussion
4. Materials and Methods
4.1. Wheat and Aphids
4.2. Evaluation of Wheat Resistance to S. avenae
4.3. Benzoxazinoids Analysis
4.4. Callose Induction and Visualization
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Xu, Z.H.; Chen, J.L.; Cheng, D.F.; Sun, J.R.; Liu, Y.; Francis, F. Discovery of English grain aphid (Hemiptera: Aphididae) biotypes in China. J. Econ. Entomol. 2011, 104, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhai, Y.; Liu, D.; Zhang, N.; Li, C.; Shi, X. Identification and genetic differentiation of Sitobion avenae (Hemiptera: Aphididae) biotypes in China. J. Econ. Entomol. 2019, 113, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hallerman, E.M.; Wu, K.; Peng, Y. Insect-Resistant genetically engineered crops in china: Development, application, and prospects for use. Annu. Rev. Entomol. 2020, 65, 273–292. [Google Scholar] [CrossRef] [Green Version]
- Aradottir, G.I.; Martin, J.L.; Clark, S.J.; Pickett, J.A.; Smart, L.E. Searching for wheat resistance to aphids and wheat bulb fly in the historical Watkins and Gediflux wheat collections. Ann. Appl. Biol. 2017, 170, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardo, C.; Singh, R.P.; Matthew, R.; Julio, H. Genetics of greenbug resistance in synthetic hexaploid wheat derived germplasm. Front. Plant Sci. 2019, 10, 782. [Google Scholar]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yactayo-Chang, J.P.; Tang, H.V.; Mendoza, J.; Christensen, S.A.; Block, A.K. Plant defense chemicals against insect pests. Agronomy 2020, 10, 1156. [Google Scholar] [CrossRef]
- Batyrshina, Z.S.; Yaakov, B.; Shavit, R.; Singh, A.; Tzin, V. Comparative transcriptomic and metabolic analysis of wild and domesticated wheat genotypes reveals differences in chemical and physical defense responses against aphids. BMC Plant Biol. 2020, 20, 19. [Google Scholar] [CrossRef] [Green Version]
- Niculaes, C.; Abramov, A.; Hannemann, L.; Frey, M. Plant protection by benzoxazinoids—Recent insights into biosynthesis and function. Agronomy 2018, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Wouters, F.C.; Blanchette, B.; Gershenzon, J.; Vassao, D.G. Plant defense and herbivore counter-defense: Benzoxazinoids and insect herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Richter, A.; Jander, G. Beyond defense: Multiple functions of benzoxazinoids in maize metabolism. Plant Cell Physiol. 2018, 59, 1528–1537. [Google Scholar] [CrossRef]
- Givovich, A.; Niemeyer, H.M. Comparison of the effect of hydroxamic acids from wheat on five species of cereal aphids. Entomol. Exp. Appl. 1995, 74, 115–119. [Google Scholar] [CrossRef]
- Argandoña, V.H.; Corcuera, L.J.; Niemeyer, H.M.; Campbell, B.C. Toxicity and feeding deterrency of hydroxamic acids from Gramineae in synthetic diets against the greenbug, Schizaphis graminum. Entomol. Exp. Appl. 1983, 34, 134–138. [Google Scholar] [CrossRef]
- Thackray, D.J.; Wrattent, S.D.; Edwards, P.J.; Niemeyer, H.M. Resistance to the aphids Sitobion avenae and Rhopalosiphum padi in Gramineae in relation to hydroxamic acid levels. Ann. Appl. Biol. 1990, 116, 573–582. [Google Scholar] [CrossRef]
- Leszczynski, B.; Dixon, A.F. Resistance of cereals to aphids: Interaction between hydroxamic acids and the aphid Sitobion avenae (Homoptera: Aphididae). Ann. Appl. Biol. 1990, 117, 21–30. [Google Scholar] [CrossRef]
- Ahmad, S.; Veyrat, N.; Gordon-Weeks, R.; Zhang, Y.; Martin, J.; Smart, L.; Glauser, G.; Erb, M.; Flors, V.; Frey, M.; et al. Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol. 2011, 157, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bing, J.W.; Guthrie, W.D.; Dicke, F.F.; Obryckp, J.J. Relation of corn leaf aphid (Homoptera: Aphididae) colonization to DIMBOA content in maize inbred lines. J. Econ. Entomol. 1990, 83, 1626–1632. [Google Scholar] [CrossRef]
- Kazemi, M.H.; Van Emden, H.F. Partial antibiosis to Rhopalosiphum padi in wheat and some phytochemical correlations. Ann. Appl. Biol. 1992, 121, 1–9. [Google Scholar] [CrossRef]
- Castaneda, L.E.; Figueroa, C.C.; Fuentes-Contreras, E.; Niemeyer, H.M.; Nespolo, R.F. Energetic costs of detoxification systems in herbivores feeding on chemically defended host plants: A correlational study in the grain aphid, Sitobion avenae. J. Exp. Biol. 2009, 212, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Elek, H.; Smart, L.; Martin, J.; Ahmad, S.; Gordon-Weeks, R.; Welham, S.; Nádasy, M.; Pickett, J.A.; Werner, C.P. The potential of hydroxamic acids in tetraploid and hexaploid wheat varieties as resistance factors against the bird-cherry oat aphid, Rhopalosiphum padi. Ann. Appl. Biol. 2013, 162, 100–109. [Google Scholar] [CrossRef]
- Pereira, J.F.; Sarria, A.L.; Powers, S.J.; Aradottir, G.I.; Caulfield, J.C.; Martin, J.; Smart, L.E.; Pickett, J.A.; Birkett, M.A.; Pereira, P.R. DIMBOA levels in hexaploid Brazilian wheat are not associated with antibiosis against the cereal aphids Rhopalosiphum padi and Sitobion avenae. Theor. Exp. Plant Physiol. 2017, 29, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Castañeda, L.E.; Figueroa, C.C.; Nespolo, R.F. Do insect pests perform better on highly defended plants? Costs and benefits of induced detoxification defences in the aphid Sitobion avenae. J. Evolution. Biol. 2010, 23, 2474–2483. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Villagrasa, M.; Eljarrat, E.; Barceló, D.; Barceló, D. Analysis of benzoxazinone derivatives in plant tissues and their degradation products in agricultural soils. Trends Analytic. Chem. 2009, 28, 1103–1114. [Google Scholar] [CrossRef]
- Mayoral, A.M.; Gutierrez, C.; Ruaz, M.L.; Castanera, P. A high performance liquid chromatography method for quantification of diboa, DIMBOA, and MBOA from aqueous extracts of corn and winter cereal plants. J. Liquid Chromatogr. Related Technol. 1994, 17, 2651–2665. [Google Scholar] [CrossRef]
- Stochmal, A.; Kus, J.; Martyniuk, S.; Oleszek, W. Concentration of benzoxazinoids in roots of field-grown wheat (Triticum aestivum L.) varieties. J. Agric. Food Chem. 2006, 54, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Finney, M.M.; Danehower, D.A.; Burton, J.D. Gas chromatographic method for the analysis of allelopathic natural products in rye (Secale cereale L.). J. Chromatogr. A 2005, 1066, 249–253. [Google Scholar] [CrossRef]
- Conceição, R.S.; Reis, I.M.A.; Cerqueira, A.P.M.; Perez, C.J.; Junior, M.C.D.S.; Branco, A.; Ifa, D.R.; Botura, M.B. Rapid structural characterisation of benzylisoquinoline and aporphine alkaloids from Ocotea spixiana acaricide extract by HPTLC-DESI-MSn. Phytochem. Anal. 2020, 31, 711–721. [Google Scholar] [CrossRef]
- Gao, L.; Shen, G.; Zhang, L.; Qi, J.; Zhang, C.; Ma, C.; Li, J.; Wang, L.; Malook, S.U.; Wu, J. An efficient system composed of maize protoplast transfection and HPLC-MS for studying the biosynthesis and regulation of maize benzoxazinoids. Plant Methods 2019, 15, 144. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.A.; Zhang, X.; Machado, R.A.; Schirmer, S.; Lori, M.; Mateo, P.; Erb, M.; Gershenzon, J. Sequestration and activation of plant toxins protect the western corn rootworm from enemies at multiple trophic levels. eLife 2017, 6, e29307. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, W.J.C.; Vincken, J.; Duran, K.; Gruppen, H. Mass spectrometric characterization of benzoxazinoid glycosides from rhizopus-elicited wheat (Triticum aestivum) seedlings. J. Agric. Food Chem. 2016, 64, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Søltoft, M.; Jørgensen, L.N.; Svensmark, B.; Fomsgaard, I.S. Benzoxazinoid concentrations show correlation with Fusarium head blight resistance in Danish wheat varieties. Biochem. Syst. Ecol. 2008, 36, 245–259. [Google Scholar] [CrossRef]
- Singh, A.; Dilkes, B.; Sela, H.; Tzin, V. The effectiveness of physical and chemical defense responses of wild emmer wheat against aphids depends on leaf position and genotype. Front. Plant Sci. 2021, 12, 667820. [Google Scholar] [CrossRef]
- Oikawa, A.; Ishihara, A.; Iwamura, H. Induction of HDMBOA-Glc accumulation and DIMBOA-Glc 4-O-methyltransferase by jasmonic acid in poaceous plants. Phytochemistry 2002, 61, 331–337. [Google Scholar] [CrossRef]
- Cambier, V.; Hance, T.; de Hoffmann, E. Variation of DIMBOA and related compounds content in relation to the age and plant organ in maize. Phytochemistry 2000, 53, 223–229. [Google Scholar] [CrossRef]
- Kowalska, I.; Kowalczyk, M. Determination of benzoxazinoids in spring and winter varieties of wheat using ultra-performance liquid chromatography coupled with mass spectrometry. Acta Chromatogr. 2019, 31, 179–182. [Google Scholar] [CrossRef]
- Campos, F.; Atkinson, J.; Arnason, J.T.; Philogene, B.; Morand, P.; Werstiuk, N.H.; Timmins, G. Toxicokinetics of 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one (DIMBOA) in the European corn borer, Ostrinia nubilalis (Hübner). J. Chem. Ecol. 1989, 15, 1989–2001. [Google Scholar] [CrossRef]
- Phuong, T.T.T.; Yamamoto, M.; Fujii, T.; Kojima, W.; Matsuo, T.; Ishikawa, Y. Comparison of the ability to catabolize DIMBOA, a maize antibiotic, between Ostrinia furnacalis and Ostrinia scapulalis (Lepidoptera: Crambidae), with reference to their hybrids. Appl. Entomol. Zool. 2016, 51, 143–149. [Google Scholar] [CrossRef]
- Tzin, V.; Lindsay, P.L.; Christensen, S.A.; Meihls, L.N.; Blue, L.B.; Jander, G. Genetic mapping shows intraspecific variation and transgressive segregation for caterpillar-induced aphid resistance in maize. Mol. Ecol. 2015, 24, 5739–5750. [Google Scholar] [CrossRef]
- Silva Brandão, K.L.; Murad, N.F.; Peruchi, A.; Martins, C.H.Z.; Omoto, C.; Figueira, A.; Brandão, M.M.; Trigo, J.R. Transcriptome differential co-expression reveals distinct molecular response of fall-armyworm strains to DIMBOA. Pest Manag. Sci. 2021, 77, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef] [PubMed]
- Argandoña, V.H.; Luza, J.G.; Niemeyer, H.M.; Corcuera, L.J. Role of hydroxamic acids in the resistance of cereals to aphids. Phytochemistry 1980, 19, 1665–1668. [Google Scholar] [CrossRef]
- Corcuera, L.J.; Queirolo, C.B.; Argandoña, V.H. Effects of 2-β-D-glucosyl-4-hydroxy-7-methoxy-1,4-benzoxazin-3-one on Schizaphis graminum (Rondani) (Insecta, Aphididae) feeding on artificial diets. Experientia 1985, 41, 514–516. [Google Scholar] [CrossRef]
- Argandoña, V.H.; Niemeyer, H.M.; Corcuera, L.J. Effect of content and distribution of hydroxamic acids in wheat on infestation by the aphid Schizaphis graminum. Phytochemistry 1981, 20, 673–676. [Google Scholar] [CrossRef]
- Elek, H.; Smart, L.; Ahmad, S.; Anda, A.; Werner, C.; Pickett, J. A comparison of the levels of hydroxamic acids in Aegilops speltoides and a hexaploid wheat and effects on Rhopalosiphum padi behaviour and fecundity. Acta Biol. Hung. 2014, 65, 38–46. [Google Scholar] [CrossRef]
- Shavit, R.; Batyrshina, Z.S.; Dotan, N.; Tzin, V. Cereal aphids differently affect benzoxazinoid levels in durum wheat. PLoS ONE 2018, 13, e208103. [Google Scholar] [CrossRef]
- Meihls, L.N.; Handrick, V.; Glauser, G.; Barbier, H.; Kaur, H.; Haribal, M.M.; Lipka, A.E.; Gershenzon, J.; Buckler, E.S.; Erb, M.; et al. Natural variation in maize aphid resistance is associated with 2,4-Dihydroxy-7-Methoxy-1,4-Benzoxazin-3-One glucoside methyltransferase activity. Plant Cell 2013, 25, 2341–2355. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Liu, H.; Zhuang, H.; Zhao, C.; Xu, Y.; Wu, S.; Qi, J.; Li, J.; Hettenhausen, C.; Wu, J. Transcriptomics and alternative splicing analyses reveal large differences between maize lines B73 and Mo17 in response to aphid Rhopalosiphum padi infestation. Front. Plant Sci. 2017, 8, 1738. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Förster, C.; Robert, C.A.M.; Züst, T.; Hu, L.; Machado, R.A.R.; Berset, J.; Handrick, V.; Knauer, T.; Hensel, G.; et al. Convergent evolution of a metabolic switch between aphid and caterpillar resistance in cereals. Sci. Adv. 2018, 4, t6797. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.S.; Keller, M.A.; Liu, X.F.; Hu, Z.Q.; Zhao, H.Y.; Liu, T.X. The resistance and correlation analysis to three species of cereal aphids (Hemiptera: Aphididae) on 10 wheat varieties or lines. J. Econ. Entomol. 2013, 106, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Pan, M.Z.; Liu, H.R.; Wang, S.Y.; Liu, T.X. Antibiosis and tolerance but not antixenosis to the grain aphid, Sitobion avenae (Hemiptera: Aphididae), are essential mechanisms of resistance in a wheat cultivar. Bull. Entomol. Res. 2015, 105, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.S.; Liu, Y.; Wang, Y.; Wang, Z.; Yu, X.; Wang, B.; Zhang, G.; Liu, X.; Hu, Z.; Zhao, H. Resistance of wheat accessions to the English grain aphid Sitobion avenae. PLoS ONE 2016, 11, e156158. [Google Scholar]
- Tu, X.; Fan, Y.; Mcneill, M.; Zhang, Z. Including predator presence in a refined model for assessing resistance of alfalfa cultivar to aphids. J. Integr. Agric. 2018, 17, 397–405. [Google Scholar] [CrossRef]






| Category | Molecular Structure | Substituent Group | Acronym | MW | ||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | ||||
| Lactams |  | H | H | H | HBOA | 165 |
| H | H | Glc | HBOA-Glc | 327 | ||
| CH3O | H | H | HMBOA | 195 | ||
| CH3O | H | Glc | HMBOA-Glc | 357 | ||
| CH3O | CH3O | H | HM2BOA | 225 | ||
| CH3O | CH3O | Glc | HM2BOA-Glc | 387 | ||
| OH | H | H | DHBOA | 181 | ||
| OH | H | Glc | DHBOA-Glc | 343 | ||
| Hydroxamic acids |  | H | H | H | DIBOA | 181 |
| H | H | Glc | DIBOA-Glc | 343 | ||
| CH3O | H | H | DIMBOA | 211 | ||
| CH3O | H | Glc | DIMBOA-Glc | 373 | ||
| CH3O | CH3O | H | DIM2BOA | 241 | ||
| CH3O | CH3O | Glc | DIM2BOA-Glc | 403 | ||
| OH | H | H | TRIBOA | 197 | ||
| OH | H | Glc | TRIBOA-Glc | 359 | ||
| Methyl derivatives |  | CH3O | H | H | HDMBOA | 225 |
| CH3O | H | Glc | HDMBOA-Glc | 387 | ||
| CH3O | CH3O | H | HDM2BOA | 255 | ||
| CH3O | CH3O | Glc | HDM2BOA-Glc | 417 | ||
| Varieties/Lines | DHBOA-Glc | HBOA-Glc | HMBOA-Glc | DIMBOA-Glc | HDMBOA-Glc | DIMBOA |
|---|---|---|---|---|---|---|
| XY6 | 91.84 ± 5.72 abcd | 433.01 ± 48.94 bc | 265.60 ± 10.74 ab | 699.55 ± 28.42 ab | 67.10 ± 9.72 bcd | 7.99 ± 0.56 def |
| XY22 | 129.50 ± 9.65 ab | 369.41 ± 52.84 bcd | 284.19 ± 16.11 ab | 669.88 ± 17.93 abc | 14.17 ± 1.54 g | 7.91 ± 0.56 def |
| XY22-3 | 65.36 ± 6.68 cde | 250.58 ± 56.20 de | 256.60 ± 27.58 ab | 754.22 ± 100.70 ab | 28.38 ± 6.46 defg | 7.58 ± 2.02 def |
| XY22-5 | 164.01 ± 11.69 a | 379.32 ± 58.29 bcd | 239.75 ± 5.21 ab | 275.77 ± 11.24 e | 140.39 ± 10.26 ab | 12.07 ± 0.76 cdef |
| 98-10-19 | 87.83 ± 11.80 abcd | 242.04 ± 41.59 de | 220.48 ± 19.50 b | 410.60 ± 113.04 cde | 43.20 ± 6.88 cdef | 13.38 ± 1.24 bcd |
| 98-10-30 | 106.89 ± 16.12 abc | 475.30 ± 63.57 ab | 151.67 ± 11.71 c | 552.50 ± 56.70 bcd | 157.03 ± 14.73 a | 6.15 ± 0.64 ef |
| TM-39 | 136.84 ± 24.73 ab | 602.67 ± 66.70 a | 230.48 ± 24.50 ab | 699.71 ± 28.45 ab | 22.33 ± 1.72 fg | 18.11 ± 1.52 abc |
| TM-47 | 117.45 ± 16.68 abc | 160.89 ± 31.87 e | 131.01 ± 8.78 c | 283.50 ± 21.64 de | 48.93 ± 9.35 bcde | 11.61 ± 0.65 cde |
| XN979 | 96.75 ± 4.59 abc | 293.99 ± 16.55 cde | 225.52 ± 29.07 ab | 423.55 ± 53.36 cde | 63.03 ± 4.97 abc | 6.32 ± 1.12 def |
| MX169 | 33.27 ± 7.63 e | 150.85 ± 10.12 e | 284.40 ± 29.78 ab | 309.70 ± 17.33 de | 37.64 ± 3.93 cdef | 4.00 ± 0.48 f |
| AK58 | 67.01 ± 6.62 bcde | 471.57 ± 56.31 ab | 150.20 ± 11.86 c | 687.25 ± 26.53 ab | 9.10 ± 0.50 h | 34.06 ± 4.36 a |
| XN1376 | 60.06 ± 4.12 de | 260.46 ± 15.07 de | 251.27 ± 32.14 ab | 806.76 ± 18.01 ab | 22.60 ± 0.39 efg | 9.05 ± 2.26 cdef |
| S122 | 61.65 ± 3.86 de | 502.18 ± 40.98 ab | 297.49 ± 27.03 a | 901.01 ± 27.48 a | 23.84 ± 1.11 efg | 19.15 ± 1.35 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Lan, H.; Cao, H.; Hu, X.; Fan, Y.; Song, Y.; Wu, L.; Liu, T.-X. Impacts of Constitutive and Induced Benzoxazinoids Levels on Wheat Resistance to the Grain Aphid (Sitobion avenae). Metabolites 2021, 11, 783. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110783
Zhang Z, Lan H, Cao H, Hu X, Fan Y, Song Y, Wu L, Liu T-X. Impacts of Constitutive and Induced Benzoxazinoids Levels on Wheat Resistance to the Grain Aphid (Sitobion avenae). Metabolites. 2021; 11(11):783. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110783
Chicago/Turabian StyleZhang, Zhanfeng, Hao Lan, Hehe Cao, Xiangshun Hu, Yongliang Fan, Yue Song, Lijuan Wu, and Tong-Xian Liu. 2021. "Impacts of Constitutive and Induced Benzoxazinoids Levels on Wheat Resistance to the Grain Aphid (Sitobion avenae)" Metabolites 11, no. 11: 783. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110783