Serum Metabolomic Analysis of Male Patients with Cannabis or Amphetamine Use Disorder
Abstract
:1. Introduction
2. Results
2.1. Effects of Cannabis and/or Amphetamine on Metabolic Profile in Patients with Amphetamine or Cannabis Use Disorders
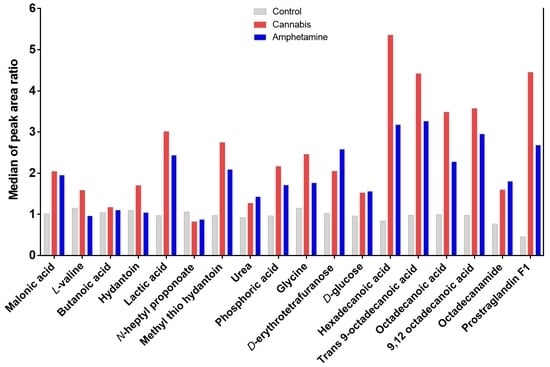
2.1.1. Effects Cannabis or Amphetamine Use Disorder on Selected Sugars
2.1.2. Effects Cannabis or Amphetamine Use Disorder on Selected Amino Acids
2.1.3. Effects of Cannabis or Amphetamine Use Disorder on Selected Fatty Acids
2.1.4. Effects of Cannabis or Amphetamine Use Disorder on Malonic Acid, Lactic Acid, and Phosphoric Acid
2.1.5. Effects of Cannabis or Amphetamine Use Disorder on Hydantoin and Methyl’ib Thio Hydantoin
2.1.6. Effects of Cannabis or Amphetamine Use Disorder on Other Metabolites
2.2. Metabolomic Profiles Analysis
2.2.1. Partial Least Squares Discriminant Analysis (PLS-DA)
2.2.2. Metabolites Analysis
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations and Informed Consent
4.2. Study Design and Blood Collection
4.3. Gas Chromatography Mass Spectroscopy (GC-MS)
4.3.1. Chemicals
4.3.2. Derivatization
4.3.3. Parameter Description
4.4. Statistical Analysis
4.5. PLS-Discriminant Analysis Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Courtney, K.E.; Ray, L.A. Methamphetamine: An update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug. Alcohol. Depend. 2014, 143, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasin, D.S. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology 2018, 43, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, Y.; Hussain, S.M.; Alnasser, S.; Almohandes, H.; Sarhandi, I. Patterns and sociodemographic characteristics of substance abuse in Al Qassim, Saudi Arabia: A retrospective study at a psychiatric rehabilitation center. Ann. Saudi Med. 2018, 38, 319. [Google Scholar] [CrossRef] [Green Version]
- Peacock, A.; Leung, J.; Larney, S.; Colledge, S.; Hickman, M.; Rehm, J.; Giovino, G.A.; West, R.; Hall, W.; Griffiths, P. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018, 113, 1905–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schifano, F.; Corkery, J.; Naidoo, V.; Oyefeso, A.; Ghodse, H. Overview of amphetamine-type stimulant mortality data–UK, 1997–2007. Neuropsychobiology 2010, 61, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatovich, D.M.; Davis, G.; Bartu, A. Morbidity associated with amphetamine-related presentations to an emergency department: A record linkage study. Emerg. Med. Australas. 2012, 24, 553–559. [Google Scholar] [CrossRef]
- Fischer, B.; Imtiaz, S.; Rudzinski, K.; Rehm, J. Crude estimates of cannabis-attributable mortality and morbidity in Canada–implications for public health focused intervention priorities. J. Public Health 2015, 38, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Bazmi, E.; Mousavi, F.; Giahchin, L.; Mokhtari, T.; Behnoush, B. Cardiovascular complications of acute amphetamine abuse: Cross-sectional study. Sultan Qaboos Univ. Med. J. 2017, 17, e31. [Google Scholar] [CrossRef]
- Foley, R.J.; Kapatkin, K.; Verani, R.; Weinman, E. Amphetamine-induced acute renal failure. South. Med. J. 1984, 77, 258–260. [Google Scholar] [CrossRef]
- Goyal, H.; Awad, H.H.; Ghali, J.K. Role of cannabis in cardiovascular disorders. J. Thorac. Dis. 2017, 9, 2079. [Google Scholar] [CrossRef] [Green Version]
- Ligeiro-Oliveira, A.P.; de Araujo, A.F.; Lazzarini, R.; Silva, Z.; De Nucci, G.; Muscará, M.N.; de Lima, W.T.; Palermo-Neto, J. Effects of amphetamine on immune-mediated lung inflammatory response in rats. Neuroimmunomodulation 2004, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Park, F.; Potukuchi, P.K.; Moradi, H.; Kovesdy, C.P. Cannabinoids and the kidney: Effects in health and disease. Am. J. Physiol. Ren. Physiol. 2017, 313, F1124–F1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almalki, A.H.; Das, S.C.; Alshehri, F.S.; Althobaiti, Y.S.; Sari, Y. Effects of sequential ethanol exposure and repeated high-dose methamphetamine on striatal and hippocampal dopamine, serotonin and glutamate tissue content in Wistar rats. Neurosci. Lett. 2018, 665, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Remião, F.; Soares, M.E.; Catarino, R.; Queiroz, G.; Bastos, M.L. d-Amphetamine-induced hepatotoxicity: Possible contribution of catecholamines and hyperthermia to the effect studied in isolated rat hepatocytes. Arch. Toxicol. 1997, 71, 429–436. [Google Scholar] [CrossRef]
- Huang, K.-L.; Shaw, K.-P.; Wang, D.; Hsu, K.; Huang, T.-S.; Chen, H.-I. Free radicals mediate amphetamine-induced acute pulmonary edema in isolated rat lung. Life Sci. 2002, 71, 1237–1244. [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, G.; Bai, Y.; Chao, J.; Chen, X.; Yao, H. Effect of methamphetamine on the fasting blood glucose in methamphetamine abusers. Metab. Brain Dis. 2018, 33, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Vanaveski, T.; Narvik, J.; Innos, J.; Philips, M.-A.; Ottas, A.; Plaas, M.; Haring, L.; Zilmer, M.; Vasar, E. Repeated Administration of D-Amphetamine Induces Distinct Alterations in Behavior and Metabolite Levels in 129Sv and Bl6 Mouse Strains. Front. Neurosci. 2018, 12, 399. [Google Scholar] [CrossRef]
- Shameem, M.; Patel, A.B. Glutamatergic and GABAergic metabolism in mouse brain under chronic nicotine exposure: Implications for addiction. PLoS ONE 2012, 7, e41824. [Google Scholar] [CrossRef] [PubMed]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572. [Google Scholar] [CrossRef] [Green Version]
- Haskins, N.; Bhuvanendran, S.; Anselmi, C.; Gams, A.; Kanholm, T.; Kocher, K.M.; LoTempio, J.; Krohmaly, K.I.; Sohai, D.; Stearrett, N. Mitochondrial Enzymes of the Urea Cycle Cluster at the Inner Mitochondrial Membrane. Front. Physiol. 2020, 11, 1753. [Google Scholar] [CrossRef]
- He, Y.; Yu, Z.; Giegling, I.; Xie, L.; Hartmann, A.; Prehn, C.; Adamski, J.; Kahn, R.; Li, Y.; Illig, T. Schizophrenia shows a unique metabolomics signature in plasma. Transl. Psychiatry 2012, 2, e149. [Google Scholar] [CrossRef] [Green Version]
- Orešič, M.; Tang, J.; Seppänen-Laakso, T.; Mattila, I.; Saarni, S.E.; Saarni, S.I.; Lönnqvist, J.; Sysi-Aho, M.; Hyötyläinen, T.; Perälä, J. Metabolome in schizophrenia and other psychotic disorders: A general population-based study. Genome Med. 2011, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bot, M.; Milaneschi, Y.; Al-Shehri, T.; Amin, N.; Garmaeva, S.; Onderwater, G.L.; Pool, R.; Thesing, C.S.; Vijfhuizen, L.S.; Vogelzangs, N. Metabolomics profile in depression: A pooled analysis of 230 metabolic markers in 5283 cases with depression and 10,145 controls. Biol. Psychiatry 2020, 87, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, H.U.; Hertel, J.; Johar, H.; Pietzner, M.; Lukaschek, K.; Atasoy, S.; Kunze, S.; Völzke, H.; Nauck, M.; Friedrich, N. A metabolome-wide association study in the general population reveals decreased levels of serum laurylcarnitine in people with depression. Mol. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, F.A.; Peeters, C.F.; Kester, M.I.; Harms, A.C.; Struys, E.A.; Hankemeier, T.; van Vlijmen, H.W.; van der Lee, S.J.; van Duijn, C.M.; Scheltens, P. Blood-based metabolic signatures in Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 8, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, M.M.; Walker, D.I.; Howell, J.C.; Watts, K.D.; Jones, D.P.; Miller, G.W.; Hu, W.T. High-resolution metabolomic profiling of Alzheimer’s disease in plasma. Ann. Clin. Transl. Neurol. 2020, 7, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, X.; Stein, T.P.; Barnes, V.; Rhodes, N.; Guo, L. Metabolic perturbance in autism spectrum disorders: A metabolomics study. J. Proteome Res. 2012, 11, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Emond, P.; Mavel, S.; Aïdoud, N.; Nadal-Desbarats, L.; Montigny, F.; Bonnet-Brilhault, F.; Barthélémy, C.; Merten, M.; Sarda, P.; Laumonnier, F. GC-MS-based urine metabolic profiling of autism spectrum disorders. Anal. Bioanal. Chem. 2013, 405, 5291–5300. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Saluja, S.; Kumar, A.; Agrawal, S.; Thind, M.; Nanda, S.; Shirani, J. Cardiovascular complications of marijuana and related substances: A review. Cardiol. Ther. 2018, 7, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.; Lewis, O.; Kumar, R.; Yeruva, S.L.H.; Curry, B.H. Amphetamine abuse related acute myocardial infarction. Case Rep. Cardiol. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.P.; Coates, A.M.; Millar, P.J.; Burr, J.F. Habitual cannabis use is associated with altered cardiac mechanics and arterial stiffness, but not endothelial function in young healthy smokers. J. Appl. Physiol. 2021, 130, 660–670. [Google Scholar] [CrossRef]
- Borini, P.; Guimarães, R.C.; Borini, S.B. Possible hepatotoxicity of chronic marijuana usage. Sao Paulo Med. J. 2004, 122, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Affas, S.; Ayas, M.F.; Kassab, I.A. Use of N-Acetylcysteine in Amphetamine-Induced Acute Liver Failure. J. Med. Cases 2021, 12, 54. [Google Scholar] [CrossRef]
- Jones, A.L.; Jarvie, D.; McDermid, G.; Proudfoot, A. Hepatocellular damage following amphetamine intoxication. J. Toxicol. Clin. Toxicol. 1994, 32, 435–444. [Google Scholar] [CrossRef]
- Steuer, A.E.; Kaelin, D.; Boxler, M.I.; Eisenbeiss, L.; Holze, F.; Vizeli, P.; Czerwinska, J.; Dargan, P.I.; Abbate, V.; Liechti, M.E. Comparative untargeted metabolomics analysis of the psychostimulants 3, 4-methylenedioxy-methamphetamine (MDMA), amphetamine, and the novel psychoactive substance mephedrone after controlled drug administration to humans. Metabolites 2020, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Krokos, A.; Deda, O.; Virgiliou, C.; Gika, H.; Raikos, N.; Aggelidou, E.; Kritis, A.; Theodoridis, G. Evaluation of Cocaine Effect on Endogenous Metabolites of HepG2 Cells Using Targeted Metabolomics. Molecules 2021, 26, 4610. [Google Scholar] [CrossRef] [PubMed]
- Roede, J.R.; Uppal, K.; Park, Y.; Lee, K.; Tran, V.; Walker, D.; Strobel, F.H.; Rhodes, S.L.; Ritz, B.; Jones, D.P. Serum metabolomics of slow vs. rapid motor progression Parkinson’s disease: A pilot study. PLoS ONE 2013, 8, e77629. [Google Scholar] [CrossRef] [Green Version]
- Skalny, A.V.; Mazaletskaya, A.L.; Zaitseva, I.P.; Skalny, A.A.; Spandidos, D.A.; Tsatsakis, A.; Lobanova, Y.N.; Skalnaya, M.G.; Aschner, M.; Tinkov, A.A. Alterations in serum amino acid profiles in children with attention deficit/hyperactivity disorder. Biomed. Rep. 2021, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Chen, Q.; Zhou, Q.; Zhang, R.; Jin, J.; Hu, F.; Liu, X.; Qin, X.; Kang, L.; Zhao, S. Characteristics of serum metabolites in sporadic amyotrophic lateral sclerosis patients based on gas chromatography-mass spectrometry. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; García, A.; García-Barrera, T.; Barbas, C.; Gómez-Ariza, J.L. Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis–mass spectrometry. Electrophoresis 2014, 35, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Mcmahon, E.M.; Andersen, D.K.; Feldman, J.M.; Schanberg, S.M. Methamphetamine-induced insulin release. Science 1971, 174, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S. Effect of cannabis on diabetic patients in rural population of udaipur region. IJSSER 2018, 3, 7303–7308. [Google Scholar]
- Bjørndal, B.; Brattelid, T.; Strand, E.; Vigerust, N.F.; Svingen, G.F.T.; Svardal, A.; Nygård, O.; Berge, R.K. Fish oil and the pan-PPAR agonist tetradecylthioacetic acid affect the amino acid and carnitine metabolism in rats. PLoS ONE 2013, 8, e66926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekhawat, P.S.; Sonne, S.; Carter, A.L.; Matern, D.; Ganapathy, V. Enzymes involved in L-carnitine biosynthesis are expressed by small intestinal enterocytes in mice: Implications for gut health. J. Crohn’s Colitis 2013, 7, e197–e205. [Google Scholar] [CrossRef]
- Fujita, Y.; Ishima, T.; Hashimoto, K. Supplementation with D-serine prevents the onset of cognitive deficits in adult offspring after maternal immune activation. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gal, N.J.; Sweeney, L.B.H.; Bennett, K.; Dahl, W. Chronic Kidney Disease: Phosphorus and Your Diet. EDIS 2020, 2016, 4. [Google Scholar]
- Li, N.; Nikaido, T.; Alireza, S.; Takagaki, T.; Chen, J.; Tagami, J. Phosphoric acid-etching promotes bond strength and formation of acid-base resistant zone on enamel. Oper. Dent. 2013, 38, 82–90. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Kang, Y.; Jeon, D.G.; Albrektsson, T. Bone reactions to oxidized titanium implants with electrochemical anion sulphuric acid and phosphoric acid incorporation. Clin. Implant Dent. Relat. Res. 2002, 4, 78–87. [Google Scholar] [CrossRef]
- Nogueira-Filho, G.R.; Todescan, S.; Shah, A.; Rosa, B.T.; Tunes, U.d.R.; Cesar Neto, J.B. Impact of cannabis sativa (marijuana) smoke on alveolar bone loss: A histometric study in rats. J. Periodontol. 2011, 82, 1602–1607. [Google Scholar] [CrossRef]
- Mosti, M.P.; Flemmen, G.; Hoff, J.; Stunes, A.K.; Syversen, U.; Wang, E. Impaired skeletal health and neuromuscular function among amphetamine users in clinical treatment. Osteoporos. Int. 2016, 27, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, H.; Shintani, D.; Ohlrogge, J. Why do mitochondria synthesize fatty acids? Evidence for involvement in lipoic acid production. Proc. Natl. Acad. Sci. USA 1997, 94, 1591–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowski, A.; Joshi, A.K.; Smith, S. Coupling of the de novo fatty acid biosynthesis and lipoylation pathways in mammalian mitochondria. J. Biol. Chem. 2007, 282, 14178–14185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef] [Green Version]
- Cori, C.F. The Glucose–Lactic Acid Cycle and Gluconeogenesis; Current Topics in Cellular Regulation; Estabrook, R.W., Srere, P., Eds.; Elsevier: Amsterdam, The Netherlans, 1981; pp. 377–387. [Google Scholar]
- Pinter, E.; Pattee, C. Fat-mobilizing action of amphetamine. J. Clin. Investig. 1968, 47, 394–402. [Google Scholar] [CrossRef]
- Ross, S.A.; ElSohly, H.N.; ElKashoury, E.A.; ElSohly, M.A. Fatty acids of cannabis seeds. Phytochem. Anal. 1996, 7, 279–283. [Google Scholar] [CrossRef]
- Babiker, E.E.; Uslu, N.; Al Juhaimi, F.; Ahmed, I.A.M.; Ghafoor, K.; Özcan, M.M.; Almusallam, I.A. Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT 2021, 139, 110537. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glac, W.; Dunacka, J.; Grembecka, B.; Świątek, G.; Majkutewicz, I.; Wrona, D. Prolonged peripheral immunosuppressive responses as consequences of random amphetamine treatment, amphetamine withdrawal and subsequent amphetamine challenges in rats. J. Neuroimmune Pharmacol. 2021, 16, 870–887. [Google Scholar] [CrossRef]
- Reddy, P.K.; Ng, T.M.; Oh, E.E.; Moady, G.; Elkayam, U. Clinical Characteristics and Management of Methamphetamine-Associated Cardiomyopathy: State-of-the-Art Review. J. Am. Heart Assoc. 2020, 9, e016704. [Google Scholar] [CrossRef]
- Ladha, K.S.; Mistry, N.; Wijeysundera, D.N.; Clarke, H.; Verma, S.; Hare, G.M.; Mazer, C.D. Recent cannabis use and myocardial infarction in young adults: A cross-sectional study. CMAJ 2021, 193, E1377–E1384. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, K.G.; Briones, M.; Thames, A.D.; Hinkin, C.H.; Zhu, T.; Wu, Y.N.; Shoptaw, S.J. Randomized, placebo-controlled trial of targeting neuroinflammation with ibudilast to treat methamphetamine use disorder. J. Neuroimmune Pharmacol. 2020, 15, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Shommu, N.S.; Jenne, C.N.; Blackwood, J.; Martin, D.-A.; Joffe, A.R.; Eccles, R.; Brindle, M.; Khanafer, I.; Vogel, H.J.; Thompson, G.C. The use of metabolomics and inflammatory mediator profiling provides a novel approach to identifying pediatric appendicitis in the emergency department. Sci. Rep. 2018, 8, 4083. [Google Scholar] [CrossRef]
- Kapoor, S.; Clay, E.; Wallace, G.R.; Fitzpatrick, M.; Bayley, R.; Young, S.P. Metabolomics in the Analysis of Inflammatory Diseases; InTech Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Rattigan, K.M.; Pountain, A.W.; Regnault, C.; Achcar, F.; Vincent, I.M.; Goodyear, C.S.; Barrett, M.P. Metabolomic profiling of macrophages determines the discrete metabolomic signature and metabolomic interactome triggered by polarising immune stimuli. PLoS ONE 2018, 13, e0194126. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Sung, M.M.; Ezekowitz, J.; Mandal, R.; Han, B.; Bjorndahl, T.C.; Bouatra, S.; Anderson, T.; Oudit, G.Y.; Wishart, D.S. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE 2015, 10, e0124844. [Google Scholar] [CrossRef]
- Ahamad, S.R.; Alhaider, A.Q.; Raish, M.; Shakeel, F. Metabolomic and elemental analysis of camel and bovine urine by GC-MS and ICP-MS. Saudi J. Biol. Sci. 2017, 24, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamad, S.R.; Raish, M.; Ahmad, A.; Shakeel, F. Potential Health Benefits and Metabolomics of Camel Milk by GC-MS and ICP-MS. Biol. Trace Elem. Res. 2017, 175, 322–330. [Google Scholar] [CrossRef] [PubMed]








| Control Group | Cann Group | Amph Group | |
|---|---|---|---|
| No. of patients | 8 | 8 | 8 |
| Gender | 8M, 0F | 8M, 0F | 8M, 0F |
| Age (Mean ± SD) | 30.38 ± 4.37 | 27.88 ± 6.83 | 31 ± 6.55 |
| Marital status | 3 married, 5 single | 2 married, 6 single | 2 married, 6 single |
| Infectious diseases (HIV, HCV, TB) | Negative | Negative | Negative |
| Substance dosage use | None | Smoking | Oral tablets |
| Substance use history 1–4 years 5–9 years 10–14 years ≥15 Years | None None None None | 1 patient 3 patient 2 patients 2 patients | 1 patient 3 patients 2 patients 2 patients |
| # | Metabolites | RT |
|---|---|---|
| 1 | Malonic acid | 6.21 |
| 2 | L-Valine | 7.34 |
| 3 | Butanoic acid | 8.21 |
| 4 | Hydantoin | 8.51 |
| 5 | Lactic acid | 9.04 |
| 6 | N-Heptyl propanoate | 9.50 |
| 7 | Methyl thio hydantoin | 11.45 |
| 8 | Urea | 11.90 |
| 9 | Phosphoric acid | 12.34 |
| 10 | Glycine | 12.68 |
| 11 | D-Erythrotetrofuranose | 20.79 |
| 12 | D-Glucose | 21.26 |
| 13 | Hexadecanoic acid | 22.87 |
| 14 | TRANS-9-Octadecenoic acid | 24.45 |
| 15 | Octadecanoic acid | 24.67 |
| 16 | 9,12-Octadecadienoic acid | 25.24 |
| 17 | Octadecenamide | 25.92 |
| 18 | Prostaglandin F1a | 28.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasmari, F.; Assiri, M.A.; Ahamad, S.R.; Aljumayi, S.R.; Alotaibi, W.H.; Alhamdan, M.M.; Alhazzani, K.; Alharbi, M.; Alqahtani, F.; Alasmari, A.F. Serum Metabolomic Analysis of Male Patients with Cannabis or Amphetamine Use Disorder. Metabolites 2022, 12, 179. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12020179
Alasmari F, Assiri MA, Ahamad SR, Aljumayi SR, Alotaibi WH, Alhamdan MM, Alhazzani K, Alharbi M, Alqahtani F, Alasmari AF. Serum Metabolomic Analysis of Male Patients with Cannabis or Amphetamine Use Disorder. Metabolites. 2022; 12(2):179. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12020179
Chicago/Turabian StyleAlasmari, Fawaz, Mohammed A. Assiri, Syed Rizwan Ahamad, Sahar R. Aljumayi, Wedad H. Alotaibi, Majd M. Alhamdan, Khalid Alhazzani, Metab Alharbi, Faleh Alqahtani, and Abdullah F. Alasmari. 2022. "Serum Metabolomic Analysis of Male Patients with Cannabis or Amphetamine Use Disorder" Metabolites 12, no. 2: 179. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12020179