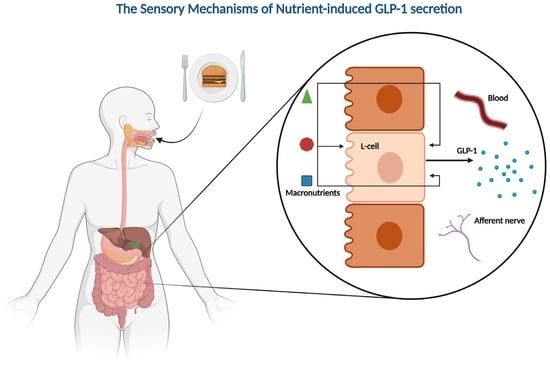
The Sensory Mechanisms of Nutrient-Induced GLP-1 Secretion
Abstract
:1. Introduction
1.1. The Endocrine Intestine
1.2. The Therapeutic Potential of GLP-1 and the Nutrient-Sensing L-Cell
2. Carbohydrate-Induced GLP-1 Secretion
3. Fat-Induced GLP-1 Secretion
4. Protein-Induced GLP-1 Secretion
5. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahlman, H.; Nilsson, O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001, 12 (Suppl. 2), S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Bucinskaite, V.; Tolessa, T.; Pedersen, J.; Rydqvist, B.; Zerihun, L.; Holst, J.J.; Hellström, P.M. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol. Motil. 2009, 21, 978-e78. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egerod, K.L.; Petersen, N.; Timshel, P.N.; Rekling, J.C.; Wang, Y.; Liu, Q.; Schwartz, T.W.; Gautron, L. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol. Metab. 2018, 12, 62–75. [Google Scholar]
- Mortensen, K.; Christensen, L.L.; Holst, J.J.; Orskov, C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003, 114, 189–196. [Google Scholar] [CrossRef]
- Egerod, K.L.; Engelstoft, M.S.; Grunddal, K.V.; Nøhr, M.K.; Secher, A.; Sakata, I.; Pedersen, J.; Windeløv, J.A.; Füchtbauer, E.M.; Olsen, J.; et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 2012, 153, 5782–5795. [Google Scholar] [CrossRef] [Green Version]
- Svendsen, B.; Pedersen, J.; Albrechtsen, N.J.; Hartmann, B.; Toräng, S.; Rehfeld, J.F.; Poulsen, S.S.; Holst, J.J. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 2015, 156, 847–857. [Google Scholar] [CrossRef]
- Beumer, J.; Artegiani, B.; Post, Y.; Reimann, F.; Gribble, F.; Nguyen, T.N.; Zeng, H.; Van den Born, M.; Van Es, J.H.; Clevers, H. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat. Cell Biol. 2018, 20, 909–916. [Google Scholar] [CrossRef]
- Lund, P.K.; Goodman, R.H.; Dee, P.C.; Habener, J.F. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc. Natl. Acad. Sci. USA 1982, 79, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Lund, P.K.; Goodman, R.H.; Montminy, M.R.; Dee, P.C.; Habener, J.F. Anglerfish islet pre-proglucagon II. Nucleotide and corresponding amino acid sequence of the cDNA. J. Biol. Chem. 1983, 258, 3280–3284. [Google Scholar] [CrossRef]
- Bell, G.I.; Santerre, R.F.; Mullenbach, G.T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 1983, 302, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.I.; Sanchez-Pescador, R.; Laybourn, P.J.; Najarian, R.C. Exon duplication and divergence in the human preproglucagon gene. Nature 1983, 304, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Mojsov, S.; Habener, J.F. Cell-specific post-translational processing of preproglucagon expressed from a metallothionein-glucagon fusion gene. J. Biol. Chem. 1986, 261, 9637–9643. [Google Scholar] [CrossRef]
- Mojsov, S.; Heinrich, G.; Wilson, I.B.; Ravazzola, M.; Orci, L.; Habener, J.F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J. Biol. Chem. 1986, 261, 11880–11889. [Google Scholar] [CrossRef]
- Orskov, C.; Holst, J.J.; Knuhtsen, S.; Baldissera, F.G.; Poulsen, S.S.; Nielsen, O.V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 1986, 119, 1467–1475. [Google Scholar] [CrossRef]
- Holst, J.J.; Orskov, C.; Nielsen, O.V.; Schwartz, T.W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987, 211, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Mojsov, S.; Weir, G.C.; Habener, J.F. Insulinotropin: Glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J. Clin. Investig. 1987, 79, 616–619. [Google Scholar] [CrossRef] [Green Version]
- Drucker, D.J.; Philippe, J.; Mojsov, S.; Chick, W.L.; Habener, J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 3434–3438. [Google Scholar] [CrossRef] [Green Version]
- Orskov, C.; Holst, J.J.; Poulsen, S.S.; Kirkegaard, P. Pancreatic and intestinal processing of proglucagon in man. Diabetologia 1987, 30, 874–881. [Google Scholar] [CrossRef]
- Kreymann, B.; Williams, G.; Ghatei, M.A.; Bloom, S.R. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet 1987, 2, 1300–1304. [Google Scholar] [CrossRef]
- Orskov, C.; Bersani, M.; Johnsen, A.H.; Højrup, P.; Holst, J.J. Complete sequences of glucagon-like peptide-1 from human and pig small intestine. J. Biol. Chem. 1989, 264, 12826–12829. [Google Scholar] [CrossRef]
- Näslund, E.; Barkeling, B.; King, N.; Gutniak, M.; Blundell, J.E.; Holst, J.J.; Rössner, S.; Hellström, P.M. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int. J. Obes. 1999, 23, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, A.; Raben, A.; Ersbøll, A.K.; Holst, J.J.; Astrup, A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int. J. Obes. 2001, 25, 781–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef]
- Korner, J.; Bessler, M.; Inabnet, W.; Taveras, C.; Holst, J.J. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg. Obes. Relat. Dis. 2007, 3, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Svane, M.S.; Bojsen-Møller, K.N.; Nielsen, S.; Jørgensen, N.B.; Dirksen, C.; Bendtsen, F.; Kristiansen, V.B.; Hartmann, B.; Holst, J.J.; Madsbad, S. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E505–E514. [Google Scholar] [CrossRef] [Green Version]
- Larraufie, P.; Roberts, G.P.; McGavigan, A.K.; Kay, R.G.; Li, J.; Leiter, A.; Melvin, A.; Biggs, E.K.; Ravn, P.; Davy, K.; et al. Important Role of the GLP-1 Axis for Glucose Homeostasis after Bariatric Surgery. Cell Rep. 2019, 26, 1399–1408.e6. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.; de Souza, A.L.; Batista, G.A.; Duran, L.F.T.; Fernandes, D.P.; Molina, V.B.C.; Gonçalves, R.; Giorgetti, J.S.; Chaim, E.A.; Alegre, S.M. GLP-1: 10-year follow-up after Roux-en-Y gastric bypass. Langenbecks Arch. Surg. 2022, 407, 559–568. [Google Scholar] [CrossRef]
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Guedes, T.P.; Martins, S.; Costa, M.; Pereira, S.S.; Morais, T.; Santos, A.; Nora, M.; Monteiro, M.P. Detailed characterization of incretin cell distribution along the human small intestine. Surg. Obes. Relat. Dis. 2015, 11, 1323–1331. [Google Scholar] [CrossRef]
- Wewer Albrechtsen, N.J.; Kuhre, R.E.; Toräng, S.; Holst, J.J. The intestinal distribution pattern of appetite- and glucose regulatory peptides in mice, rats and pigs. BMC Res. Notes 2016, 9, 60. [Google Scholar]
- Orskov, C.; Wettergren, A.; Holst, J.J. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand. J. Gastroenterol. 1996, 31, 665–670. [Google Scholar] [PubMed]
- Ahlkvist, L.; Vikman, J.; Pacini, G.; Ahrén, B. Synergism by individual macronutrients explains the marked early GLP-1 and islet hormone responses to mixed meal challenge in mice. Regul. Pept. 2012, 178, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qualmann, C.; Nauck, M.A.; Holst, J.J.; Orskov, C.; Creutzfeldt, W. Glucagon-like peptide 1 (7-36 amide) secretion in response to luminal sucrose from the upper and lower gut. A study using alpha-glucosidase inhibition (acarbose). Scand. J. Gastroenterol. 1995, 30, 892–896. [Google Scholar] [CrossRef]
- Ritzel, U.; Fromme, A.; Ottleben, M.; Leonhardt, U.; Ramadori, G. Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol. 1997, 34, 18–21. [Google Scholar] [CrossRef]
- Reimann, F.; Gribble, F.M. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes 2002, 51, 2757–2763. [Google Scholar] [CrossRef] [Green Version]
- Gribble, F.M.; Williams, L.; Simpson, A.K.; Reimann, F. A Novel Glucose-Sensing Mechanism Contributing to Glucagon-Like Peptide-1 Secretion from the GLUTag Cell Line. Diabetes 2003, 52, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose Sensing in L Cells: A Primary Cell Study. Cell Metab. 2008, 8, 532–539. [Google Scholar]
- Moriya, R.; Shirakura, T.; Ito, J.; Mashiko, S.; Seo, T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1358–E1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhre, R.E.; Frost, C.R.; Svendsen, B.; Holst, J.J. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes 2015, 64, 370–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, E.W.; de Fontgalland, D.; Rabbitt, P.; Hollington, P.; Sposato, L.; Due, S.L.; Wattchow, D.A.; Rayner, C.K.; Deane, A.M.; Young, R.L.; et al. Mechanisms Controlling Glucose-Induced GLP-1 Secretion in Human Small Intestine. Diabetes 2017, 66, 2144–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [Green Version]
- van der Beek, C.M.; Canfora, E.E.; Lenaerts, K.; Troost, F.J.; Damink, S.; Holst, J.J.; Masclee, A.A.M.; Dejong, C.H.C.; Blaak, E.E. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. 2016, 130, 2073–2082. [Google Scholar] [CrossRef]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; Dejong, C.H.C.; Blaak, E.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [Green Version]
- Elliott, R.M.; Morgan, L.M.; Tredger, J.A.; Deacon, S.; Wright, J.; Marks, V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: Acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993, 138, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Göke, R.; Richter, G.; Fehmann, H.C.; Arnold, R.; Göke, B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 1995, 56, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Feinle, C.; O’Donovan, D.; Doran, S.; Andrews, J.M.; Wishart, J.; Chapman, I.; Horowitz, M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G798–G807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilichiewicz, A.; O’Donovan, D.; Feinle, C.; Lei, Y.; Wishart, J.M.; Bryant, L.; Meyer, J.H.; Horowitz, M.; Jones, K.L. Effect of lipase inhibition on gastric emptying of, and the glycemic and incretin responses to, an oil/aqueous drink in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 3829–3834. [Google Scholar] [CrossRef] [Green Version]
- Ellrichmann, M.; Kapelle, M.; Ritter, P.R.; Holst, J.J.; Herzig, K.H.; Schmidt, W.E.; Schmitz, F.; Meier, J.J. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7-36)-amide-1, cholecystokinin, and peptide YY concentrations. J. Clin. Endocrinol. Metab. 2008, 93, 3995–3998. [Google Scholar] [CrossRef] [Green Version]
- Beglinger, S.; Drewe, J.; Schirra, J.; Göke, B.; D’Amato, M.; Beglinger, C. Role of fat hydrolysis in regulating glucagon-like Peptide-1 secretion. J. Clin. Endocrinol. Metab. 2010, 95, 879–886. [Google Scholar] [CrossRef]
- Beysen, C.; Karpe, F.; Fielding, B.A.; Clark, A.; Levy, J.C.; Frayn, K.N. Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia 2002, 45, 1533–1541. [Google Scholar]
- Thomsen, C.; Rasmussen, O.; Lousen, T.; Holst, J.J.; Fenselau, S.; Schrezenmeir, J.; Hermansen, K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am. J. Clin. Nutr. 1999, 69, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Rocca, A.S.; Brubaker, P.L. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology 1995, 136, 5593–5599. [Google Scholar] [CrossRef]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R524–R533. [Google Scholar] [CrossRef] [Green Version]
- Kiyasu, J.Y.; Bloom, B.; Chaikoff, I.L. The portal transport of absorbed fatty acids. J. Biol. Chem. 1952, 199, 415–419. [Google Scholar] [CrossRef]
- Lu, W.J.; Yang, Q.; Yang, L.; Lee, D.; D’Alessio, D.; Tso, P. Chylomicron formation and secretion is required for lipid-stimulated release of incretins GLP-1 and GIP. Lipids 2012, 47, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psichas, A.; Larraufie, P.F.; Goldspink, D.A.; Gribble, F.M.; Reimann, F. Chylomicrons stimulate incretin secretion in mouse and human cells. Diabetologia 2017, 60, 2475–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [Green Version]
- Christensen, L.W.; Kuhre, R.E.; Janus, C.; Svendsen, B.; Holst, J.J. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol. Rep. 2015, 3, e12551. [Google Scholar] [CrossRef] [Green Version]
- Hauge, M.; Vestmar, M.A.; Husted, A.S.; Ekberg, J.P.; Wright, M.J.; Di Salvo, J.; Weinglass, A.B.; Engelstoft, M.S.; Madsen, A.N.; Lückmann, M.; et al. GPR40 (FFAR1)—Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol. Metab. 2015, 4, 3–14. [Google Scholar] [CrossRef]
- Leifke, E.; Naik, H.; Wu, J.; Viswanathan, P.; DeManno, D.; Kipnes, M.; Vakilynejad, M. A Multiple-Ascending-Dose Study to Evaluate Safety, Pharmacokinetics, and Pharmacodynamics of a Novel GPR40 Agonist, TAK-875, in Subjects with Type 2 Diabetes. Clin. Pharmacol. Ther. 2012, 92, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Kaku, K.; Enya, K.; Nakaya, R.; Ohira, T.; Matsuno, R. Long-term safety and efficacy of fasiglifam (TAK-875), a G-protein-coupled receptor 40 agonist, as monotherapy and combination therapy in Japanese patients with type 2 diabetes: A 52-week open-label phase III study. Diabetes Obes. Metab. 2016, 18, 925–929. [Google Scholar] [CrossRef]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef]
- Lauffer, L.M.; Iakoubov, R.; Brubaker, P.L. GPR119 Is Essential for Oleoylethanolamide-Induced Glucagon-Like Peptide-1 Secretion From the Intestinal Enteroendocrine L-Cell. Diabetes 2009, 58, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Hansen, K.B.; Rosenkilde, M.M.; Knop, F.K.; Wellner, N.; Diep, T.A.; Rehfeld, J.F.; Andersen, U.B.; Holst, J.J.; Hansen, H.S. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. 2011, 96, E1409–E1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekberg, J.H.; Hauge, M.; Kristensen, L.V.; Madsen, A.N.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.; Egerod, K.L.; Timshel, P.; Kowalski, T.J.; et al. GPR119, a Major Enteroendocrine Sensor of Dietary Triglyceride Metabolites Coacting in Synergy with FFA1 (GPR40). Endocrinology 2016, 157, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Mace, O.J.; Tough, I.R.; White, J.; Cock, T.A.; Warpman Berglund, U.; Schindler, M.; Cox, H.M. Gastrointestinal hormonal responses on GPR119 activation in lean and diseased rodent models of type 2 diabetes. Int. J. Obes. 2014, 38, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.; Buning, C.; Halland, N.; Pöverlein, C.; Schwink, L. G Protein-Coupled Receptor 119 (GPR119) Agonists for the Treatment of Diabetes: Recent Progress and Prevailing Challenges. J. Med. Chem. 2016, 59, 3579–3592. [Google Scholar] [CrossRef]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales. Front. Pharmacol. 2021, 12, 807548. [Google Scholar] [CrossRef]
- Skov, A.R.; Toubro, S.; Rønn, B.; Holm, L.; Astrup, A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Nijs, I.; van Ooijen, M.; Kovacs, E.M. High protein intake sustains weight maintenance after body weight loss in humans. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Latner, J.D.; Schwartz, M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite 1999, 33, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Poppitt, S.D.; McCormack, D.; Buffenstein, R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol. Behav. 1998, 64, 279–285. [Google Scholar] [CrossRef]
- Crovetti, R.; Porrini, M.; Santangelo, A.; Testolin, G. The influence of thermic effect of food on satiety. Eur. J. Clin. Nutr. 1998, 52, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geliebter, A.A. Effects of equicaloric loads of protein, fat, and carbohydrate on food intake in the rat and man. Physiol. Behav. 1979, 22, 267–273. [Google Scholar] [CrossRef]
- Raben, A.; Agerholm-Larsen, L.; Flint, A.; Holst, J.J.; Astrup, A. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am. J. Clin. Nutr. 2003, 77, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejeune, M.P.; Westerterp, K.R.; Adam, T.C.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef] [Green Version]
- Cordier-Bussat, M.; Bernard, C.; Levenez, F.; Klages, N.; Laser-Ritz, B.; Philippe, J.; Chayvialle, J.A.; Cuber, J.C. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes 1998, 47, 1038–1045. [Google Scholar] [CrossRef]
- Modvig, I.M.; Kuhre, R.E.; Holst, J.J. Peptone-mediated glucagon-like peptide-1 secretion depends on intestinal absorption and activation of basolaterally located Calcium-Sensing Receptors. Physiol. Rep. 2019, 7, e14056. [Google Scholar] [CrossRef]
- Matsumura, K.; Miki, T.; Jhomori, T.; Gonoi, T.; Seino, S. Possible role of PEPT1 in gastrointestinal hormone secretion. Biochem. Biophys. Res. Commun. 2005, 336, 1028–1032. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Leoncini, R.; De Col, A.; Tamini, S.; Cicolini, S.; Abbruzzese, L.; Cella, S.G.; Sartorio, A. The Appetite-Suppressant and GLP-1-Stimulating Effects of Whey Proteins in Obese Subjects are Associated with Increased Circulating Levels of Specific Amino Acids. Nutrients 2020, 12, 775. [Google Scholar] [CrossRef] [Green Version]
- Reimann, F.; Williams, L.; da Silva Xavier, G.; Rutter, G.A.; Gribble, F.M. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 2004, 47, 1592–1601. [Google Scholar] [CrossRef] [Green Version]
- Tolhurst, G.; Zheng, Y.; Parker, H.E.; Habib, A.M.; Reimann, F.; Gribble, F.M. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 2011, 152, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oya, M.; Kitaguchi, T.; Pais, R.; Reimann, F.; Gribble, F.; Tsuboi, T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J. Biol. Chem. 2013, 288, 4513–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamshah, A.; Spreckley, E.; Norton, M.; Kinsey-Jones, J.S.; Amin, A.; Ramgulam, A.; Cao, Y.; Johnson, R.; Saleh, K.; Akalestou, E.; et al. l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int. J. Obes. 2017, 41, 1693–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudenko, O.; Shang, J.; Munk, A.; Ekberg, J.P.; Petersen, N.; Engelstoft, M.S.; Egerod, K.L.; Hjorth, S.A.; Wu, M.; Feng, Y.; et al. The aromatic amino acid sensor GPR142 controls metabolism through balanced regulation of pancreatic and gut hormones. Mol. Metab. 2019, 19, 49–64. [Google Scholar] [CrossRef]
- Modvig, I.M.; Kuhre, R.E.; Jepsen, S.L.; Xu, S.F.S.; Engelstoft, M.S.; Egerod, K.L.; Schwartz, T.W.; Ørskov, C.; Rosenkilde, M.M.; Holst, J.J. Amino acids differ in their capacity to stimulate GLP-1 release from the perfused rat small intestine and stimulate secretion by different sensing mechanisms. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E874–E885. [Google Scholar] [CrossRef]
- Greenfield, J.R.; Farooqi, I.S.; Keogh, J.M.; Henning, E.; Habib, A.M.; Blackwood, A.; Reimann, F.; Holst, J.J.; Gribble, F.M. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am. J. Clin. Nutr. 2009, 89, 106–113. [Google Scholar] [CrossRef]
- Clemmensen, C.; Jørgensen, C.V.; Smajilovic, S.; Bräuner-Osborne, H. Robust GLP-1 secretion by basic L-amino acids does not require the GPRC6A receptor. Diabetes Obes. Metab. 2017, 19, 599–603. [Google Scholar] [CrossRef]
- Amin, A.; Neophytou, C.; Thein, S.; Martin, N.M.; Alamshah, A.; Spreckley, E.; Bloom, S.R.; Murphy, K.G. L-Arginine Increases Postprandial Circulating GLP-1 and PYY Levels in Humans. Obesity 2018, 26, 1721–1726. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hjørne, A.P.; Modvig, I.M.; Holst, J.J. The Sensory Mechanisms of Nutrient-Induced GLP-1 Secretion. Metabolites 2022, 12, 420. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12050420
Hjørne AP, Modvig IM, Holst JJ. The Sensory Mechanisms of Nutrient-Induced GLP-1 Secretion. Metabolites. 2022; 12(5):420. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12050420
Chicago/Turabian StyleHjørne, Anna Pii, Ida Marie Modvig, and Jens Juul Holst. 2022. "The Sensory Mechanisms of Nutrient-Induced GLP-1 Secretion" Metabolites 12, no. 5: 420. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12050420