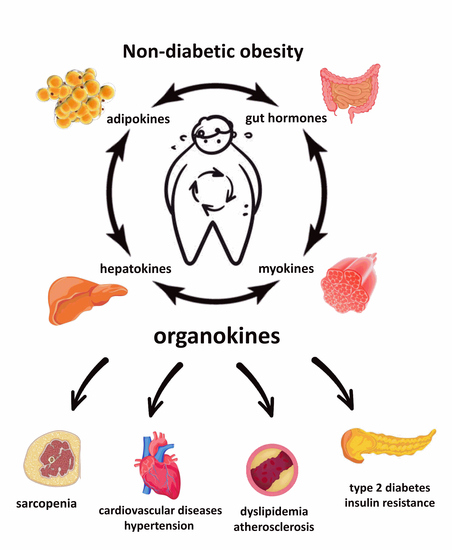
Crucial Regulatory Role of Organokines in Relation to Metabolic Changes in Non-Diabetic Obesity
Abstract
:1. Introduction
2. Role of Novel Adipokines in Non-Diabetic Obesity
3. Role of Novel Hepatokines and Gastrointestinal Hormones in Non-Diabetic Obesity
4. Role of Myokines in Non-Diabetic Obesity
5. Treatment and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahmoud, I.; Al-Wandi, A.S.; Gharaibeh, S.S.; Mohamed, S.A. Concordances and correlations between anthropometric indices of obesity: A systematic review. Public Health 2021, 198, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.O.; Almandoz, J.P.; Frias, J.P.; Galindo, R.J. Obesity among Latinx people in the United States: A review. Obesity 2023, 31, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Mamdouh, H.; Hussain, H.Y.; Ibrahim, G.M.; Alawadi, F.; Hassanein, M.; Zarooni, A.A.; Suwaidi, H.A.; Hassan, A.; Alsheikh-Ali, A.; Alnakhi, W.K. Prevalence and associated risk factors of overweight and obesity among adult population in Dubai: A population-based cross-sectional survey in Dubai, the United Arab Emirates. BMJ Open 2023, 13, e062053. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, E.; Fismen, A.S.; Helleve, A.; Hongoro, C.; Sewpaul, R.; Reddy, P.; Alaba, O.; Harbron, J. Trends in prevalence of overweight and obesity among South African and European adolescents: A comparative outlook. BMC Public Health 2022, 22, 2287. [Google Scholar] [CrossRef] [PubMed]
- Gallus, S.; Lugo, A.; Murisic, B.; Bosetti, C.; Boffetta, P.; La Vecchia, C. Overweight and obesity in 16 European countries. Eur. J. Nutr. 2015, 54, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, D.V.; Karatzi, K.; Kantaras, P.; Liatis, S.; Iotova, V.; Bazdraska, Y.; Tankova, T.; Cardon, G.; Wikström, K.; Rurik, I.; et al. Prevalence and Socioeconomic Correlates of Adult Obesity in Europe: The Feel4Diabetes Study. Int. J. Environ. Res. Public Health 2022, 19, 12572. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.; Baranchi, M.; Goodarzi, M.T. The implication of hepatokines in metabolic syndrome. Diabetes Metab. Syndr. 2019, 13, 2477–2480. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, P.; Harangi, M.; Seres, I.; Paragh, G. Paraoxonase-1 and adipokines: Potential links between obesity and atherosclerosis. Chem. Biol. Interact. 2016, 259, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef]
- de Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef]
- Caleyachetty, R.; Thomas, G.N.; Toulis, K.A.; Mohammed, N.; Gokhale, K.M.; Balachandran, K.; Nirantharakumar, K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J. Am. Coll. Cardiol. 2017, 70, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Joseph, F. Adipose tissue and adipokines: The association with and application of adipokines in obesity. Scientifica 2014, 2014, 328592. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Zhang, H.; Sairam, M.R. Sex hormone imbalances and adipose tissue dysfunction impacting on metabolic syndrome; a paradigm for the discovery of novel adipokines. Horm. Mol. Biol. Clin. Investig. 2014, 17, 89–97. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Fotiadis, G.; Lambadiari, V.; Maratou, E.; Dimitriadis, G.; Liapis, C.D. Serum levels of novel adipokines in patients with acute ischemic stroke: Potential contribution to diagnosis and prognosis. Peptides 2014, 57, 12–16. [Google Scholar] [CrossRef]
- Su, X.; Peng, D. Adipokines as novel biomarkers of cardio-metabolic disorders. Clin. Chim. Acta 2020, 507, 31–38. [Google Scholar] [CrossRef]
- Zhao, L.; Leung, L.L.; Morser, J. Chemerin Forms: Their Generation and Activity. Biomedicines 2022, 10, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Eisenberg, D.; Zhao, L.; Adams, C.; Leib, R.; Morser, J.; Leung, L. Chemerin activation in human obesity. Obesity 2016, 24, 1522–1529. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: Tumor necrosis factor-α stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg. Obes. Relat. Dis. 2013, 9, 306–314. [Google Scholar] [CrossRef]
- Wójcik, M.; Kozioł-Kozakowska, A.; Januś, D.; Furtak, A.; Małek, A.; Sztefko, K.; Starzyk, J.B. Circulating chemerin level may be associated with early vascular pathology in obese children without overt arterial hypertension—Preliminary results. J. Pediatr. Endocrinol. Metab. 2020, 33, 729–734. [Google Scholar] [CrossRef]
- Wen, J.; Wang, J.; Guo, L.; Cai, W.; Wu, Y.; Chen, W.; Tang, X. Chemerin stimulates aortic smooth muscle cell proliferation and migration via activation of autophagy in VSMCs of metabolic hypertension rats. Am. J. Transl. Res. 2019, 11, 1327–1342. [Google Scholar] [PubMed]
- Ferland, D.J.; Mullick, A.E.; Watts, S.W. Chemerin as a Driver of Hypertension: A Consideration. Am. J. Hypertens. 2020, 33, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Lavis, P.; Morra, S.; Orte Cano, C.; Albayrak, N.; Corbière, V.; Olislagers, V.; Dauby, N.; Del Marmol, V.; Marchant, A.; Decaestecker, C.; et al. Chemerin plasma levels are increased in COVID-19 patients and are an independent risk factor of mortality. Front. Immunol. 2022, 13, 941663. [Google Scholar] [CrossRef]
- Lőrincz, H.; Katkó, M.; Harangi, M.; Somodi, S.; Gaál, K.; Fülöp, P.; Paragh, G.; Seres, I. Strong correlations between circulating chemerin levels and lipoprotein subfractions in nondiabetic obese and nonobese subjects. Clin. Endocrinol. 2014, 81, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Spiroglou, S.G.; Kostopoulos, C.G.; Varakis, J.N.; Papadaki, H.H. Adipokines in periaortic and epicardial adipose tissue: Differential expression and relation to atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 115–130. [Google Scholar] [CrossRef]
- Davidson, W.S.; Heink, A.; Sexmith, H.; Dolan, L.M.; Gordon, S.M.; Otvos, J.D.; Melchior, J.T.; Elder, D.A.; Khoury, J.; Geh, E.; et al. Obesity is associated with an altered HDL subspecies profile among adolescents with metabolic disease. J. Lipid. Res. 2017, 58, 1916–1923. [Google Scholar] [CrossRef] [Green Version]
- Mertens, A.; Holvoet, P. Oxidized LDL and HDL: Antagonists in atherothrombosis. FASEB J. 2001, 15, 2073–2084. [Google Scholar] [CrossRef]
- Fülöp, P.; Seres, I.; Lőrincz, H.; Harangi, M.; Somodi, S.; Paragh, G. Association of chemerin with oxidative stress, inflammation and classical adipokines in non-diabetic obese patients. J. Cell Mol. Med. 2014, 18, 1313–1320. [Google Scholar] [CrossRef]
- Barnard, S.A.; Pieters, M.; De Lange, Z. The contribution of different adipose tissue depots to plasma plasminogen activator inhibitor-1 (PAI-1) levels. Blood Rev. 2016, 30, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Raiko, J.R.; Oikonen, M.; Wendelin-Saarenhovi, M.; Siitonen, N.; Kähönen, M.; Lehtimäki, T.; Viikari, J.; Jula, A.; Loo, B.M.; Huupponen, R.; et al. Plasminogen activator inhitor-1 associates with cardiovascular risk factors in healthy young adults in the Cardiovascular Risk in Young Finns Study. Atherosclerosis 2012, 224, 208–212. [Google Scholar] [CrossRef]
- Lee, M.H.; Hammad, S.M.; Semler, A.J.; Luttrell, L.M.; Lopes-Virella, M.F.; Klein, R.L. HDL3, but not HDL2, stimulates plasminogen activator inhibitor-1 release from adipocytes: The role of sphingosine-1-phosphate. J. Lipid Res. 2010, 51, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Somodi, S.; Seres, I.; Lőrincz, H.; Harangi, M.; Fülöp, P.; Paragh, G. Plasminogen Activator Inhibitor-1 Level Correlates with Lipoprotein Subfractions in Obese Nondiabetic Subjects. Int. J. Endocrinol. 2018, 2018, 9596054. [Google Scholar] [CrossRef]
- Dawson, S.J.; Wiman, B.; Hamsten, A.; Green, F.; Humphries, S.; Henney, A.M. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J. Biol. Chem. 1993, 268, 10739–10745. [Google Scholar] [CrossRef] [PubMed]
- Roncal, C.; Orbe, J.; Belzunce, M.; Rodríguez, J.A.; Páramo, J.A. The 4G/5G PAI-1 polymorphism influences the endothelial response to IL-1 and the modulatory effect of pravastatin. J. Thromb. Haemost. 2006, 4, 1798–1803. [Google Scholar] [CrossRef]
- Eriksson, P.; Nilsson, L.; Karpe, F.; Hamsten, A. Very-low-density lipoprotein response element in the promoter region of the human plasminogen activator inhibitor-1 gene implicated in the impaired fibrinolysis of hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 20–26. [Google Scholar] [CrossRef]
- Nilsson, L.; Banfi, C.; Diczfalusy, U.; Tremoli, E.; Hamsten, A.; Eriksson, P. Unsaturated fatty acids increase plasminogen activator inhibitor-1 expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1679–1685. [Google Scholar] [CrossRef] [Green Version]
- Olufadi, R.; Byrne, C.D. Effects of VLDL and remnant particles on platelets. Pathophysiol. Haemost. Thromb. 2006, 35, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, H.; Galgóczy, E.; Katkó, M.; Ratku, B.; Ötvös, T.; Harangi, M.; Paragh, G.; Szabó, Z.; Somodi, S. Plasminogen activator inhibitor-1 level associated with the components of metabolic syndrome in a 4G/5G polymorphism dependent manner. Atherosclerosis. 2022, 355, 166. [Google Scholar] [CrossRef]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef] [PubMed]
- El Sehmawy, A.A.; Diab, F.E.A.; Hassan, D.A.; Mohammed, D.S.; Gamal El Din Al Anany, M.; Eldesoky, N.A.; Elamir, R.Y. Utility of Adipokines and IL-10 in Association with Anthropometry in Prediction of Insulin Resistance in Obese Children. Diabetes Metab. Syndr. Obes. 2022, 15, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Z.; Ye, Z.; Li, Q.; Wen, J.; Tao, X.; Chen, L.; He, M.; Wang, X.; Lu, B.; et al. Lipocalin-2, glucose metabolism and chronic low-grade systemic inflammation in Chinese people. Cardiovasc. Diabetol. 2012, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Tanabe, Y.; Kaneko, K. Increased lipocalin 2 levels in adolescents with type 2 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2021, 34, 979–985. [Google Scholar] [CrossRef]
- Şen, S.; Özalp Kızılay, D.; Taneli, F.; Özen, Ç.; Ertan, P.; Özunan, İ.; Yıldız, R.; Ersoy, B. Urinary NGAL is a Potential Biomarker for Early Renal Injury in Insulin Resistant Obese Non-diabetic Children. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 400–407. [Google Scholar] [CrossRef]
- Polidori, N.; Giannini, C.; Salvatore, R.; Pelliccia, P.; Parisi, A.; Chiarelli, F.; Mohn, A. Role of urinary NGAL and KIM-1 as biomarkers of early kidney injury in obese prepubertal children. J. Pediatr. Endocrinol. Metab. 2020, 33, 1183–1189. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.D.; Feng, Y.M.; Huang, Z.Q.; Xie, Y.B.; Zhou, J.; Li, J. Relationship between morning peak phenomenon and early renal injury NGAL in H-type hypertension. Blood Press. 2022, 31, 200–206. [Google Scholar] [CrossRef]
- Atashak, S.; Stannard, S.R.; Daraei, A.; Soltani, M.; Saeidi, A.; Moradi, F.; Laher, I.; Hackney, A.C.; Zouhal, H. High-intensity Interval Training Improves Lipocalin-2 and Omentin-1 Levels in Men with Obesity. Int. J. Sports Med. 2022, 43, 328–335. [Google Scholar] [CrossRef]
- Ponzetti, M.; Aielli, F.; Ucci, A.; Cappariello, A.; Lombardi, G.; Teti, A.; Rucci, N. Lipocalin 2 increases after high-intensity exercise in humans and influences muscle gene expression and differentiation in mice. J. Cell. Physiol. 2022, 237, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Steele, F.R.; Chader, G.J.; Johnson, L.V.; Tombran-Tink, J. Pigment epithelium-derived factor: Neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc. Natl. Acad. Sci. USA 1993, 90, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.; Wu, L.E.; Economou, C.; Turpin, S.M.; Matzaris, M.; Hoehn, K.L.; Hevener, A.L.; James, D.E.; Duh, E.J.; Watt, M.J. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab. 2009, 10, 40–47. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Quan, X.; Qin, X.; Zhou, Y.; Liu, Z.; Chao, Z.; Jia, C.; Qin, H.; Zhang, H. Pigment epithelium-derived factor and its role in microvascular-related diseases. Biochimie 2022, 200, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Chen, K.D.; Hsu, L.W.; Kung, C.P.; Li, S.R.; Chen, C.C.; Chiu, K.W.; Goto, S.; Chen, C.L. Decreased PEDF Promotes Hepatic Fatty Acid Uptake and Lipid Droplet Formation in the Pathogenesis of NAFLD. Nutrients 2020, 12, 270. [Google Scholar] [CrossRef]
- Toloza, F.J.K.; Pérez-Matos, M.C.; Ricardo-Silgado, M.L.; Morales-Álvarez, M.C.; Mantilla-Rivas, J.O.; Pinzón-Cortés, J.A.; Pérez-Mayorga, M.; Arévalo-García, M.L.; Tolosa-González, G.; Mendivil, C.O. Comparison of plasma pigment epithelium-derived factor (PEDF), retinol binding protein 4 (RBP-4), chitinase-3-like protein 1 (YKL-40) and brain-derived neurotrophic factor (BDNF) for the identification of insulin resistance. J. Diabetes Complicat. 2017, 31, 1423–1429. [Google Scholar] [CrossRef]
- Tolusso, B.; Gigante, M.R.; Alivernini, S.; Petricca, L.; Fedele, A.L.; Di Mario, C.; Aquilanti, B.; Magurano, M.R.; Ferraccioli, G.; Gremese, E. Chemerin and PEDF Are Metaflammation-Related Biomarkers of Disease Activity and Obesity in Rheumatoid Arthritis. Front. Med. 2018, 5, 207. [Google Scholar] [CrossRef]
- Karasek, D.; Spurna, J.; Kubickova, V.; Krystynik, O.; Cibickova, L.; Schovanek, J.; Goldmannova, D. Association of pigment epithelium derived factor with von Willebrand factor and plasminogen activator inhibitor 1 in patients with type 2 diabetes. Physiol. Res. 2019, 68, 409–418. [Google Scholar] [CrossRef]
- Matsui, T.; Nishino, Y.; Ojima, A.; Maeda, S.; Tahara, N.; Yamagishi, S.I. Pigment epithelium-derived factor improves metabolic derangements and ameliorates dysregulation of adipocytokines in obese type 2 diabetic rats. Am. J. Pathol. 2014, 184, 1094–1103. [Google Scholar] [CrossRef]
- Daniel, R.; He, Z.; Carmichael, K.P.; Halper, J.; Bateman, A. Cellular localization of gene expression for progranulin. J. Histochem. Cytochem. 2000, 48, 999–1009. [Google Scholar] [CrossRef]
- Tang, W.; Lu, Y.; Tian, Q.Y.; Zhang, Y.; Guo, F.J.; Liu, G.Y.; Syed, N.M.; Lai, Y.; Lin, E.A.; Kong, L.; et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 2011, 332, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Konopka, J.; Liu, C. Insights into the role of progranulin in immunity, infection, and inflammation. J. Leukoc. Biol. 2013, 93, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Deng, H.; Hu, Z. Plasma progranulin concentrations are increased in patients with type 2 diabetes and obesity and correlated with insulin resistance. Mediat. Inflamm. 2013, 2013, 360190. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Sutaih, R.H.; Kamfar, H.Z.; Alagha, A.E.; Marzouki, Z.M. Serum progranulin levels in relation to insulin resistance in childhood obesity. J. Pediatr. Endocrinol. Metab. 2017, 30, 1251–1256. [Google Scholar] [CrossRef]
- Wang, F.; Chen, T.; Sun, L.; Lv, H.; Li, X.; Shen, J.; Chen, L.; Chu, Z.; Hou, M. Circulating PGRN Levels Are Increased but Not Associated with Insulin Sensitivity or. Dis. Markers 2018, 2018, 3729402. [Google Scholar] [CrossRef]
- Safarzade, A.; Alizadeh, H.; Bastani, Z. The effects of circuit resistance training on plasma progranulin level, insulin resistance and body composition in obese men. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20190050. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Mukheja, S.; Varma, S.; Kalra, H.S.; Khosa, B.S.; Vohra, K. Serum progranulin/tumor necrosis factor-α ratio as independent predictor of systolic blood pressure in overweight hypertensive patients: A cross-sectional study. Egypt Heart J. 2020, 72, 25. [Google Scholar] [CrossRef]
- Nádró, B.; Lőrincz, H.; Molnár, Á.; Szentpéteri, A.; Zöld, E.; Seres, I.; Páll, D.; Paragh, G.; Kempler, P.; Harangi, M.; et al. Effects of alpha-lipoic acid treatment on serum progranulin levels and inflammatory markers in diabetic neuropathy. J. Int. Med. Res. 2021, 49, 3000605211012213. [Google Scholar] [CrossRef]
- Brock, J.; Schmid, A.; Karrasch, T.; Pfefferle, P.; Schlegel, J.; Busse, I.; Hauenschild, A.; Schmidt, B.; Koukou, M.; Arapogianni, E.; et al. Progranulin serum levels and gene expression in subcutaneous vs visceral adipose tissue of severely obese patients undergoing bariatric surgery. Clin. Endocrinol. 2019, 91, 400–410. [Google Scholar] [CrossRef]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, J. Vaspin: A novel serpin with insulin-sensitizing effects. Expert Opin. Investig. Drugs 2008, 17, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Serinkan Cinemre, F.B.; Cinemre, H.; Bahtiyar, N.; Kahyaoğlu, B.; Ağaç, M.T.; Shundo, H.; Sevinç, L.; Aydemir, B. Apelin, Omentin-1, and Vaspin in patients with essential hypertension: Association of adipokines with trace elements, inflammatory cytokines, and oxidative damage markers. Ir. J. Med. Sci. 2021, 190, 97–106. [Google Scholar] [CrossRef]
- Taheri, E.; Hosseini, S.; Qorbani, M.; Mirmiran, P. Association of adipocytokines with lipid and glycemic profiles in women with normal weight obesity. BMC Endocr. Disord. 2020, 20, 171. [Google Scholar] [CrossRef]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 88–94. [Google Scholar] [CrossRef]
- Özkan, E.A.; Sadigov, A.; Öztürk, O. Evaluation of Serum Omentin-1, Vaspin, Leptin, Adiponectin Levels in Obese/Overweight Children and Their Relationship With Non-Alcoholic Fatty Liver Disease. Clin. Nutr. Res. 2022, 11, 194–203. [Google Scholar] [CrossRef]
- Baig, M.; Gazzaz, Z.J.; Bakarman, M.A.; Alzahrani, S.H. Correlation of Serum Vaspin, Omentin-1, and adiponectin with metabolic phenotypes in Type-2 diabetes mellitus patients. Pak. J. Med. Sci. 2021, 37, 1762–1767. [Google Scholar] [CrossRef]
- Yang, R.Z.; Lee, M.J.; Hu, H.; Pray, J.; Wu, H.B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- Zengi, S.; Zengi, O.; Kirankaya, A.; Kucuk, S.H.; Kutanis, E.E.; Yigit, O. Serum omentin-1 levels in obese children. J. Pediatr. Endocrinol. Metab. 2019, 32, 247–251. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Kaur, H.; Adams-Huet, B.; Bremer, A.A. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E514–E517. [Google Scholar] [CrossRef]
- Eimal Latif, A.H.; Anwar, S.; Gautham, K.S.; Kadurei, F.; Ojo, R.O.; Hafizyar, F.; Muhammad Haroon, D.; Rakesh, F.; Talpur, A.S. Association of Plasma Omentin-1 Levels With Diabetes and Its Complications. Cureus 2021, 13, e18203. [Google Scholar] [CrossRef]
- Cetin Sanlialp, S.; Nar, G.; Nar, R. Relationship between circulating serum omentin-1 levels and nascent metabolic syndrome in patients with hypertension. J. Investig. Med. 2022, 70, 780–785. [Google Scholar] [CrossRef]
- Himani, K.; Vani, G.; Mishra, S.; Mahdi, A.A.; Shally, A. Association of serum Interleukin-10, omentin-1 and visfatin concentration with metabolic risk factors in obese children. Diabetes Metab. Syndr. 2019, 13, 2069–2074. [Google Scholar] [CrossRef]
- Siegrist, M.; Heitkamp, M.; Braun, I.; Vogg, N.; Haller, B.; Langhof, H.; Koenig, W.; Halle, M. Changes of omentin-1 and chemerin during 4 weeks of lifestyle intervention and 1 year follow-up in children with obesity. Clin. Nutr. 2021, 40, 5648–5654. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Kassimis, G.; Patsourakos, N.; Kanonidis, I.; Valsami, G. Omentin-1 and vaspin serum levels in patients with pre-clinical carotid atherosclerosis and the effect of statin therapy on them. Cytokine 2021, 138, 155364. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef]
- Jung, T.W.; Pyun, D.H.; Kim, T.J.; Lee, H.J.; Park, E.S.; Abd El-Aty, A.M.; Hwang, E.J.; Shin, Y.K.; Jeong, J.H. Meteorin-like protein (METRNL)/IL-41 improves LPS-induced inflammatory responses via AMPK or PPARδ-mediated signaling pathways. Adv. Med. Sci. 2021, 66, 155–161. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, Y.E.; Kim, J.M.; Choung, S.; Joung, K.H.; Kim, H.J.; Ku, B.J. Serum Meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 135, 7–10. [Google Scholar] [CrossRef]
- Wang, R.; Hu, D.; Zhao, X.; Hu, W. Correlation of serum meteorin-like concentrations with diabetic nephropathy. Diabetes Res. Clin. Pract. 2020, 169, 108443. [Google Scholar] [CrossRef]
- Wang, K.; Li, F.; Wang, C.; Deng, Y.; Cao, Z.; Cui, Y.; Xu, K.; Ln, P.; Sun, Y. Serum Levels of Meteorin-Like (Metrnl) Are Increased in Patients with Newly Diagnosed Type 2 Diabetes Mellitus and Are Associated with Insulin Resistance. Med. Sci. Monit. 2019, 25, 2337–2343. [Google Scholar] [CrossRef]
- Dieplinger, H.; Dieplinger, B. Afamin--A pleiotropic glycoprotein involved in various disease states. Clin. Chim. Acta 2015, 446, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Melmer, A.; Fineder, L.; Lamina, C.; Kollerits, B.; Dieplinger, B.; Braicu, I.; Sehouli, J.; Cadron, I.; Vergote, I.; Mahner, S.; et al. Plasma concentrations of the vitamin E-binding protein afamin are associated with overall and progression-free survival and platinum sensitivity in serous ovarian cancer—A study by the OVCAD consortium. Gynecol. Oncol. 2013, 128, 38–43. [Google Scholar] [CrossRef]
- Song, H.J.; Xue, Y.L.; Qiu, Z.L.; Luo, Q.Y. Comparative serum proteomic analysis identified afamin as a downregulated protein in papillary thyroid carcinoma patients with non-131I-avid lung metastases. Nucl. Med. Commun. 2013, 34, 1196–1203. [Google Scholar] [CrossRef]
- Wang, W.K.; Tsai, C.H.; Liu, Y.W.; Lai, C.C.; Huang, C.C.; Sheen-Chen, S.M. Afamin expression in breast cancer. Asian J. Surg. 2020, 43, 750–754. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.; Lee, S.Y.; Park, S.Y.; Dieplinger, H.; Yea, K.; Lee, S.H.; Koh, J.M.; Kim, G.S. Afamin stimulates osteoclastogenesis and bone resorption via Gi-coupled receptor and Ca2+/calmodulin-dependent protein kinase (CaMK) pathways. J. Endocrinol. Investig. 2013, 36, 876–882. [Google Scholar] [CrossRef]
- Kononikhin, A.S.; Zakharova, N.V.; Semenov, S.D.; Bugrova, A.E.; Brzhozovskiy, A.G.; Indeykina, M.I.; Fedorova, Y.B.; Kolykhalov, I.V.; Strelnikova, P.A.; Ikonnikova, A.Y.; et al. Prognosis of Alzheimer’s Disease Using Quantitative Mass Spectrometry of Human Blood Plasma Proteins and Machine Learning. Int. J. Mol. Sci. 2022, 23, 7907. [Google Scholar] [CrossRef]
- Köninger, A.; Iannaccone, A.; Hajder, E.; Frank, M.; Schmidt, B.; Schleussner, E.; Kimmig, R.; Gellhaus, A.; Dieplinger, H. Afamin predicts gestational diabetes in polycystic ovary syndrome patients preconceptionally. Endocr. Connect. 2019, 8, 616–624. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Kovanicova, Z.; Zemkova, E.; Kuzma, M.; Belan, V.; Payer, J.; Gasperikova, D.; Dieplinger, H.; Ukropcova, B.; et al. Serum Afamin a Novel Marker of Increased Hepatic Lipid Content. Front. Endocrinol. 2021, 12, 670425. [Google Scholar] [CrossRef]
- Kollerits, B.; Lamina, C.; Huth, C.; Marques-Vidal, P.; Kiechl, S.; Seppälä, I.; Cooper, J.; Hunt, S.C.; Meisinger, C.; Herder, C.; et al. Plasma Concentrations of Afamin Are Associated With Prevalent and Incident Type 2 Diabetes: A Pooled Analysis in More Than 20,000 Individuals. Diabetes Care 2017, 40, 1386–1393. [Google Scholar] [CrossRef]
- Stakhneva, E.M.; Kashtanova, E.V.; Polonskaya, Y.V.; Striukova, E.V.; Shramko, V.S.; Sadovski, E.V.; Kurguzov, A.V.; Murashov, I.S.; Chernyavskii, A.M.; Ragino, Y.I. The Search for Associations of Serum Proteins with the Presence of Unstable Atherosclerotic Plaque in Coronary Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 12795. [Google Scholar] [CrossRef]
- Pitkänen, N.; Finkenstedt, A.; Lamina, C.; Juonala, M.; Kähönen, M.; Mäkelä, K.M.; Dieplinger, B.; Viveiros, A.; Melmer, A.; Leitner, I.; et al. Afamin predicts the prevalence and incidence of nonalcoholic fatty liver disease. Clin. Chem. Lab. Med. 2022, 60, 243–251. [Google Scholar] [CrossRef]
- Juhász, I.; Ujfalusi, S.; Seres, I.; Lőrincz, H.; Varga, V.E.; Paragh, G.; Somodi, S.; Harangi, M. Afamin Levels and Their Correlation with Oxidative and Lipid Parameters in Non-diabetic, Obese Patients. Biomolecules 2022, 12, 116. [Google Scholar] [CrossRef]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef]
- Rychter, A.M.; Skrzypczak-Zielińska, M.; Zielińska, A.; Eder, P.; Souto, E.B.; Zawada, A.; Ratajczak, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity? Int. J. Mol. Sci. 2020, 21, 5229. [Google Scholar] [CrossRef]
- Fan, J.; Yin, S.; Lin, D.; Liu, Y.; Chen, N.; Bai, X.; Ke, Q.; Shen, J.; You, L.; Lin, X.; et al. Association of Serum Retinol-Binding Protein 4 Levels and the Risk of Incident Type 2 Diabetes in Subjects With Prediabetes. Diabetes Care 2019, 42, 1574–1581. [Google Scholar] [CrossRef]
- Schiborn, C.; Weber, D.; Grune, T.; Biemann, R.; Jäger, S.; Neu, N.; Müller von Blumencron, M.; Fritsche, A.; Weikert, C.; Schulze, M.B.; et al. Retinol and Retinol Binding Protein 4 Levels and Cardiometabolic Disease Risk. Circ. Res. 2022, 131, 637–649. [Google Scholar] [CrossRef]
- Nono Nankam, P.A.; Blüher, M. Retinol-binding protein 4 in obesity and metabolic dysfunctions. Mol. Cell. Endocrinol. 2021, 531, 111312. [Google Scholar] [CrossRef]
- Xiang, J.; Dai, H.; Hou, Y.; Wang, Q.; Wang, T.; Li, M.; Zhao, Z.; Lu, J.; Dai, M.; Zhang, D.; et al. Sexual Dimorphism in the Association of Serum Retinol-Binding Protein-4 With Long-Term Dynamic Metabolic Profiles in Non-Diabetes. Front. Endocrinol. 2022, 13, 880467. [Google Scholar] [CrossRef]
- Korek, E.; Gibas-Dorna, M.; Chęcińska-Maciejewska, Z.; Krauss, H.; Łagiedo-Żelazowska, M.; Kołodziejczak, B.; Bogdański, P. Serum RBP4 positively correlates with triglyceride level but not with BMI, fat mass and insulin resistance in healthy obese and non-obese individuals. Biomarkers 2018, 23, 683–688. [Google Scholar] [CrossRef]
- Lőrincz, H.; Csige, I.; Harangi, M.; Szentpéteri, A.; Seres, I.; Szabó, Z.; Paragh, G.; Somodi, S. Low Levels of Serum Fetuin-A and Retinol-Binding Protein 4 Correlate with Lipoprotein Subfractions in Morbid Obese and Lean Non-Diabetic Subjects. Life 2021, 11, 881. [Google Scholar] [CrossRef]
- Kwanbunjan, K.; Panprathip, P.; Phosat, C.; Chumpathat, N.; Wechjakwen, N.; Puduang, S.; Auyyuenyong, R.; Henkel, I.; Schweigert, F.J. Association of retinol binding protein 4 and transthyretin with triglyceride levels and insulin resistance in rural thais with high type 2 diabetes risk. BMC Endocr. Disord. 2018, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.N.; Teran-Garcia, M. From infancy to aging: Biological and behavioral modifiers of Fetuin-A. Biochimie 2016, 124, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Biomarkers of vascular calcification in serum. Adv. Clin. Chem. 2020, 98, 91–147. [Google Scholar] [CrossRef]
- Himmetoglu, S.; Teksoz, S.; Zengin, K.; Yesim, T.; Taskın, M.; Dincer, Y. Serum levels of fetuin A and 8-hydroxydeoxyguanosine in morbidly obese subjects. Exp. Clin. Endocrinol. Diabetes 2013, 121, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Ahsan, N.; Nasim, A.; Alam, F. Association of fetuin-A with dyslipidemia and insulin resistance in type-II Diabetics of Pakistani population. Pak. J. Med. Sci. 2020, 36, 64–68. [Google Scholar] [CrossRef]
- Dogru, T.; Genc, H.; Tapan, S.; Aslan, F.; Ercin, C.N.; Ors, F.; Kara, M.; Sertoglu, E.; Karslioglu, Y.; Bagci, S.; et al. Plasma fetuin-A is associated with endothelial dysfunction and subclinical atherosclerosis in subjects with nonalcoholic fatty liver disease. Clin. Endocrinol. 2013, 78, 712–717. [Google Scholar] [CrossRef]
- Dadej, D.; Szczepanek-Parulska, E.; Ruchała, M. Interplay between Fatty Acid Binding Protein 4, Fetuin-A, Retinol Binding Protein 4 and Thyroid Function in Metabolic Dysregulation. Metabolites 2022, 12, 300. [Google Scholar] [CrossRef]
- Pan, X.; Wen, S.W.; Bestman, P.L.; Kaminga, A.C.; Acheampong, K.; Liu, A. Fetuin-A in Metabolic syndrome: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0229776. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cai, W.J.; Chang, X.Y.; Li, J.; Su, X.H.; Zhu, L.Y.; Wang, X.L.; Sun, K. Association between fetuin-A levels with insulin resistance and carotid intima-media thickness in patients with new-onset type 2 diabetes mellitus. Biomed. Rep. 2014, 2, 839–842. [Google Scholar] [CrossRef]
- Ismail, N.A.; Ragab, S.; El Dayem, S.M.; Elbaky, A.A.; Salah, N.; Hamed, M.; Assal, H.; Koura, H. Fetuin-A levels in obesity: Differences in relation to metabolic syndrome and correlation with clinical and laboratory variables. Arch. Med. Sci. 2012, 8, 826–833. [Google Scholar] [CrossRef]
- Khadir, A.; Kavalakatt, S.; Madhu, D.; Hammad, M.; Devarajan, S.; Tuomilehto, J.; Tiss, A. Fetuin-A levels are increased in the adipose tissue of diabetic obese humans but not in circulation. Lipids Health Dis. 2018, 17, 291. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi-Noori, E.; Salehi, N.; Mozafari, H.; Elieh Ali Komi, D.; Saidi, M.; Bahrehmand, F.; Vaisi-Raygani, A.; Elahirad, S.; Moini, A.; Kiani, A. Association of AHSG gene polymorphisms with serum Fetuin-A levels in individuals with cardiovascular calcification in west of Iran. Mol. Biol. Rep. 2020, 47, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- de Haan, A.; Ahmadizar, F.; van der Most, P.J.; Thio, C.H.L.; Kamali, Z.; Ani, A.; Ghanbari, M.; Chaker, L.; van Meurs, J.; Ikram, M.K.; et al. Genetic Determinants of Serum Calcification Propensity and Cardiovascular Outcomes in the General Population. Front. Cardiovasc. Med. 2021, 8, 809717. [Google Scholar] [CrossRef]
- Rubinow, K.B.; Henderson, C.M.; Robinson-Cohen, C.; Himmelfarb, J.; de Boer, I.H.; Vaisar, T.; Kestenbaum, B.; Hoofnagle, A.N. Kidney function is associated with an altered protein composition of high-density lipoprotein. Kidney Int. 2017, 92, 1526–1535. [Google Scholar] [CrossRef]
- Huang, J.; Lee, H.; Zivkovic, A.M.; Smilowitz, J.T.; Rivera, N.; German, J.B.; Lebrilla, C.B. Glycomic analysis of high density lipoprotein shows a highly sialylated particle. J. Proteome Res. 2014, 13, 681–691. [Google Scholar] [CrossRef]
- Kailemia, M.J.; Wei, W.; Nguyen, K.; Beals, E.; Sawrey-Kubicek, L.; Rhodes, C.; Zhu, C.; Sacchi, R.; Zivkovic, A.M.; Lebrilla, C.B. Targeted Measurements of O- and N-Glycopeptides Show That Proteins in High Density Lipoprotein Particles Are Enriched with Specific Glycosylation Compared to Plasma. J. Proteome Res. 2018, 17, 834–845. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Sepa-Kishi, D.M.; Ceddia, R.B. Circulating fibroblast growth factor 21 is reduced, whereas its production is increased in a fat depot-specific manner in cold-acclimated rats. Adipocyte 2018, 7, 238–247. [Google Scholar] [CrossRef]
- Gong, Q.; Hu, Z.; Zhang, F.; Cui, A.; Chen, X.; Jiang, H.; Gao, J.; Han, Y.; Liang, Q.; Ye, D.; et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 2016, 64, 425–438. [Google Scholar] [CrossRef]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Yu, R.T.; Mangelsdorf, D.J.; Kliewer, S.A. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, X.; Weiszmann, J.; Johnstone, S.; Wang, W.; Yu, X.; Romanow, W.; Thibault, S.; Li, Y.; Wang, Z. Agonistic β-Klotho antibody mimics fibroblast growth factor 21 (FGF21) functions. J. Biol. Chem. 2018, 293, 14678–14688. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, Y.B.; Hu, H. Metabolic role of fibroblast growth factor 21 in liver, adipose and nervous system tissues. Biomed. Rep. 2017, 6, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Chen, L.; Li, Q.; Zhao, Y.; Yin, D.; Sun, S. Relationship between Serum FGF21 and vWF Expression and Carotid Atherosclerosis in Elderly Patients with Hypertension. J. Healthc. Eng. 2022, 2022, 6777771. [Google Scholar] [CrossRef]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.; Chow, W.S.; Tso, A.W.; Lam, K.S.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef]
- Molnár, Á.; Szentpéteri, A.; Lőrincz, H.; Seres, I.; Harangi, M.; Balogh, Z.; Kempler, P.; Paragh, G.; Sztanek, F. Change of Fibroblast Growth Factor 21 Level Correlates with the Severity of Diabetic Sensory Polyneuropathy after Six-Week Physical Activity. Rev. Cardiovasc. Med. 2022, 23, 160. [Google Scholar] [CrossRef]
- Haghighi, A.H.; Hajinia, M.; Askari, R.; Abbasian, S.; Goldfied, G. Effect of high-intensity interval training and high-intensity resistance training on irisin and fibroblast growth factor 21 in men with overweight and obesity. Can. J. Physiol. Pharmacol. 2022, 100, 937–944. [Google Scholar] [CrossRef]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Green, B.D.; Grieve, D.J. Biochemical properties and biological actions of obestatin and its relevence in type 2 diabetes. Peptides 2018, 100, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Polidori, N.; Neri, C.R.; D’Adamo, E.; Chiarelli, F.; Mohn, A. Gut Hormones Secretion across Clusters of Metabolic Syndrome in Prepubertal Children with Obesity. Horm. Res. Paediatr. 2022, 95, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Aly, G.S.; Hassan, N.E.; Anwar, G.M.; Ahmed, H.H.; El-Masry, S.A.; El-Banna, R.A.; Ahmed, N.H.; Kamal, A.N.; Tarkan, R.S. Ghrelin, obestatin and the ghrelin/obestatin ratio as potential mediators for food intake among obese children: A case control study. J. Pediatr. Endocrinol. Metab. 2020, 33, 199–204. [Google Scholar] [CrossRef]
- Egido, E.M.; Hernández, R.; Marco, J.; Silvestre, R.A. Effect of obestatin on insulin, glucagon and somatostatin secretion in the perfused rat pancreas. Regul. Pept. 2009, 152, 61–66. [Google Scholar] [CrossRef]
- Granata, R.; Settanni, F.; Gallo, D.; Trovato, L.; Biancone, L.; Cantaluppi, V.; Nano, R.; Annunziata, M.; Campiglia, P.; Arnoletti, E.; et al. Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function. Diabetes 2008, 57, 967–979. [Google Scholar] [CrossRef]
- Agnew, A.; Calderwood, D.; Chevallier, O.P.; Greer, B.; Grieve, D.J.; Green, B.D. Chronic treatment with a stable obestatin analog significantly alters plasma triglyceride levels but fails to influence food intake; fluid intake; body weight; or body composition in rats. Peptides 2011, 32, 755–762. [Google Scholar] [CrossRef]
- Grala, T.M.; Kay, J.K.; Walker, C.G.; Sheahan, A.J.; Littlejohn, M.D.; Lucy, M.C.; Roche, J.R. Expression analysis of key somatotropic axis and liporegulatory genes in ghrelin- and obestatin-infused dairy cows. Domest. Anim. Endocrinol. 2010, 39, 76–83. [Google Scholar] [CrossRef]
- Szentpéteri, A.; Lőrincz, H.; Somodi, S.; Varga, V.E.; Paragh, G.; Seres, I.; Harangi, M. Serum obestatin level strongly correlates with lipoprotein subfractions in non-diabetic obese patients. Lipids Health Dis. 2018, 17, 39. [Google Scholar] [CrossRef]
- Mishra, A.K.; Dubey, V.; Ghosh, A.R. Obesity: An overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism 2016, 65, 48–65. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Taylor, M.S.; Hwang, Y.; Hsiao, P.Y.; Boeke, J.D.; Cole, P.A. Ghrelin O-acyltransferase assays and inhibition. Methods Enzymol. 2012, 514, 205–228. [Google Scholar] [CrossRef]
- Mani, B.K.; Osborne-Lawrence, S.; Mequinion, M.; Lawrence, S.; Gautron, L.; Andrews, Z.B.; Zigman, J.M. The role of ghrelin-responsive mediobasal hypothalamic neurons in mediating feeding responses to fasting. Mol. Metab. 2017, 6, 882–896. [Google Scholar] [CrossRef]
- Druce, M.R.; Wren, A.M.; Park, A.J.; Milton, J.E.; Patterson, M.; Frost, G.; Ghatei, M.A.; Small, C.; Bloom, S.R. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. 2005, 29, 1130–1136. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.R.; Wood, C.M.; Kunos, G. Recent progress in the discovery of ghrelin. RSC Med. Chem. 2020, 11, 1136–1144. [Google Scholar] [CrossRef]
- Ibrahim Abdalla, M.M. Ghrelin—Physiological Functions and Regulation. Eur. Endocrinol. 2015, 11, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M.; Uhr, M.; Kraus, T. Evaluation of the comparability of commercial ghrelin assays. Clin. Chem. 2004, 50, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef]
- Dam, T.T.; Peters, K.W.; Fragala, M.; Cawthon, P.M.; Harris, T.B.; McLean, R.; Shardell, M.; Alley, D.E.; Kenny, A.; Ferrucci, L.; et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wong, P.Y.; Chung, Y.L.; Chow, S.K.; Cheung, W.H.; Law, S.W.; Chan, J.C.N.; Wong, R.M.Y. Deciphering the “obesity paradox” in the elderly: A systematic review and meta-analysis of sarcopenic obesity. Obes. Rev. 2022, 24, e13534. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Nettleship, J.E.; Pugh, P.J.; Channer, K.S.; Jones, T.; Jones, R.D. Inverse relationship between serum levels of interleukin-1beta and testosterone in men with stable coronary artery disease. Horm. Metab. Res. 2007, 39, 366–371. [Google Scholar] [CrossRef]
- Mudali, S.; Dobs, A.S. Effects of testosterone on body composition of the aging male. Mech. Ageing Dev. 2004, 125, 297–304. [Google Scholar] [CrossRef]
- Abdulnour, J.; Doucet, E.; Brochu, M.; Lavoie, J.M.; Strychar, I.; Rabasa-Lhoret, R.; Prud’homme, D. The effect of the menopausal transition on body composition and cardiometabolic risk factors: A Montreal-Ottawa New Emerging Team group study. Menopause 2012, 19, 760–767. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef]
- Teufel, A.; Malik, N.; Mukhopadhyay, M.; Westphal, H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene 2002, 297, 79–83. [Google Scholar] [CrossRef]
- Ferrer-Martinez, A.; Ruiz-Lozano, P.; Chien, K.R. Mouse PeP: A novel peroxisomal protein linked to myoblast differentiation and development. Dev. Dyn. 2002, 224, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Triantafyllou, G.A.; Fernandez-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belen Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Becerril, S.; Mendez-Gimenez, L.; Ramirez, B.; Sainz, N.; Catalan, V.; Gomez-Ambrosi, J.; Fruhbeck, G. Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int. J. Obes. 2015, 39, 397–407. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010, 285, 7153–7164. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, Q.; Liu, J.; Jia, S. Irisin, an exercise-induced myokine as a metabolic regulator: An updated narrative review. Diabetes Metab. Res. Rev. 2016, 32, 51–59. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernandez-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef]
- Mu, J.; Brozinick, J.T., Jr.; Valladares, O.; Bucan, M.; Birnbaum, M.J. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 2001, 7, 1085–1094. [Google Scholar] [CrossRef]
- Shi, X.; Lin, M.; Liu, C.; Xiao, F.; Liu, Y.; Huang, P.; Zeng, X.; Yan, B.; Liu, S.; Li, X.; et al. Elevated circulating irisin is associated with lower risk of insulin resistance: Association and path analyses of obese Chinese adults. BMC Endocr. Disord. 2016, 16, 44. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, M.K.; Bae, K.H.; Seo, H.A.; Jeong, J.Y.; Lee, W.K.; Kim, J.G.; Lee, I.K.; Park, K.G. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 96–101. [Google Scholar] [CrossRef]
- Liu, J.J.; Wong, M.D.; Toy, W.C.; Tan, C.S.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Sesti, G.; Andreozzi, F.; Fiorentino, T.V.; Mannino, G.C.; Sciacqua, A.; Marini, M.A.; Perticone, F. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014, 51, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef]
- Ansari, S.; Djalali, M.; Mohammadzadeh Honarvar, N.; Mazaherioun, M.; Zarei, M.; Agh, F.; Gholampour, Z.; Javanbakht, M.H. The Effect of n-3 Polyunsaturated Fatty Acids Supplementation on Serum Irisin in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Endocrinol. Metab. 2017, 15, e40614. [Google Scholar] [CrossRef]
- Oelmann, S.; Nauck, M.; Volzke, H.; Bahls, M.; Friedrich, N. Circulating Irisin Concentrations Are Associated with a Favourable Lipid Profile in the General Population. PLoS ONE 2016, 11, e0154319. [Google Scholar] [CrossRef] [Green Version]
- White, T.A.; LeBrasseur, N.K. Myostatin and sarcopenia: Opportunities and challenges—A mini-review. Gerontology 2014, 60, 289–293. [Google Scholar] [CrossRef]
- Pahlavani, H.A. Exercise-induced signaling pathways to counteracting cardiac apoptotic processes. Front. Cell. Dev. Biol. 2022, 10, 950927. [Google Scholar] [CrossRef]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef]
- Leger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008, 11, 163–175B. [Google Scholar] [CrossRef]
- Motahari Rad, M.; Bijeh, N.; Attarzadeh Hosseini, S.R.; Raouf Saeb, A. The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2020, 1–10. [Google Scholar] [CrossRef]
- Shimasaki, S.; Koga, M.; Esch, F.; Cooksey, K.; Mercado, M.; Koba, A.; Ueno, N.; Ying, S.Y.; Ling, N.; Guillemin, R. Primary structure of the human follistatin precursor and its genomic organization. Proc. Natl. Acad. Sci. USA 1988, 85, 4218–4222. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Kalista, S.; Lause, P.; Tsuchida, K.; Thissen, J.P. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E157–E164. [Google Scholar] [CrossRef]
- Nakatani, M.; Takehara, Y.; Sugino, H.; Matsumoto, M.; Hashimoto, O.; Hasegawa, Y.; Murakami, T.; Uezumi, A.; Takeda, S.; Noji, S.; et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 2008, 22, 477–487. [Google Scholar] [CrossRef]
- Mendell, J.R.; Sahenk, Z.; Malik, V.; Gomez, A.M.; Flanigan, K.M.; Lowes, L.P.; Alfano, L.N.; Berry, K.; Meadows, E.; Lewis, S.; et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol. Ther. 2015, 23, 192–201. [Google Scholar] [CrossRef]
- Singh, R.; Bhasin, S.; Braga, M.; Artaza, J.N.; Pervin, S.; Taylor, W.E.; Krishnan, V.; Sinha, S.K.; Rajavashisth, T.B.; Jasuja, R. Regulation of myogenic differentiation by androgens: Cross talk between androgen receptor/ beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology 2009, 150, 1259–1268. [Google Scholar] [CrossRef] [Green Version]
- Braga, M.; Bhasin, S.; Jasuja, R.; Pervin, S.; Singh, R. Testosterone inhibits transforming growth factor-beta signaling during myogenic differentiation and proliferation of mouse satellite cells: Potential role of follistatin in mediating testosterone action. Mol. Cell. Endocrinol. 2012, 350, 39–52. [Google Scholar] [CrossRef]
- Singh, R.; Braga, M.; Reddy, S.T.; Lee, S.J.; Parveen, M.; Grijalva, V.; Vergnes, L.; Pervin, S. Follistatin Targets Distinct Pathways To Promote Brown Adipocyte Characteristics in Brown and White Adipose Tissues. Endocrinology 2017, 158, 1217–1230. [Google Scholar] [CrossRef]
- Salem, A.M.; Latif, R.; Rafique, N.; Aldawlan, M.I.; Almulla, L.B.; Alghirash, D.Y.; Fallatah, O.A.; Alotaibi, F.M.; Aljabbari, F.H.; Yar, T. Variations of Ghrelin and Obestatin Hormones During the Menstrual Cycle of Women of Different BMIs. Int. J. Womens Health 2022, 14, 1297–1305. [Google Scholar] [CrossRef]
- Liu, Y.; Douglas, P.S.; Lip, G.Y.H.; Thabane, L.; Li, L.; Ye, Z.; Li, G. Relationship between obesity severity, metabolic status and cardiovascular disease in obese adults. Eur. J. Clin. Investig. 2022, 53, e13912. [Google Scholar] [CrossRef]
- April-Sanders, A.K.; Rodriguez, C.J. Metabolically Healthy Obesity Redefined. JAMA Netw. Open 2021, 4, e218860. [Google Scholar] [CrossRef]
- Guzmán-García, J.M.; Romero-Saldaña, M.; Molina-Recio, G.; Álvarez-Fernández, C.; Raya-Cano, E.; Molina-Luque, R. Diagnostic accuracy of the waist-to-height ratio and other anthropometric indices for metabolically healthy obesity in the working population. Front. Nutr. 2022, 9, 962054. [Google Scholar] [CrossRef]
- Hernández-Pinto, A.; Polato, F.; Subramanian, P.; Rocha-Muñoz, A.; Vitale, S.; de la Rosa, E.J.; Becerra, S.P. PEDF peptides promote photoreceptor survival in rd10 retina models. Exp. Eye Res. 2019, 184, 24–29. [Google Scholar] [CrossRef]
- Rühlmann, C.; Dannehl, D.; Brodtrück, M.; Adams, A.C.; Stenzel, J.; Lindner, T.; Krause, B.J.; Vollmar, B.; Kuhla, A. Neuroprotective Effects of the FGF21 Analogue LY2405319. J. Alzheimers. Dis. 2021, 80, 357–369. [Google Scholar] [CrossRef]
- Kim, A.M.; Somayaji, V.R.; Dong, J.Q.; Rolph, T.P.; Weng, Y.; Chabot, J.R.; Gropp, K.E.; Talukdar, S.; Calle, R.A. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes. Metab. 2017, 19, 1762–1772. [Google Scholar] [CrossRef]
- Charles, E.D.; Neuschwander-Tetri, B.A.; Pablo Frias, J.; Kundu, S.; Luo, Y.; Tirucherai, G.S.; Christian, R. Pegbelfermin (BMS-986036), PEGylated FGF21, in Patients with Obesity and Type 2 Diabetes: Results from a Randomized Phase 2 Study. Obesity 2019, 27, 41–49. [Google Scholar] [CrossRef] [Green Version]





| Organokine | Putative Role in Obesity and Obesity-Related Disturbances | Change | Reference |
|---|---|---|---|
| Adipokines | |||
| Chemerin | Mediation of obesity-associated low-grade inflammation; Negative correlation with HDL-linked antioxidant paraoxonase-1 enzyme; Strong correlation with the markers of dyslipidemia including lipoprotein subfractions; Biomarker for obesity and insulin resistance; Mechanistic influence on systolic blood pressure and hypertension. | ↑ | [23,26,28,32] |
| PAI-1 | Thrombotic effects and inflammation; Biomarker for obesity, insulin resistance and T2DM; Associations with the markers of atherogenic dyslipidemia (correlations with HDL subfractions and ApoAI; PAI-1 release stimulated by small-sized HDL in adipocytes; VLDL was capable of increasing the PAI-1 level in endothelial cells). | ↑ | [33,35,36,41] |
| LCN-2 | Biomarker for early renal injury; Biomarker for insulin resistance; Biomarker for hypertension; High LCN-2 levels negatively affect muscle physiology. | ↑ | [45,46,49,51] |
| PEDF | Anti-angiogenic properties by direct effects on endothelial cells; Role in lipid metabolism by the binding of adipose triglyceride lipase; PEDF expression may be upregulated by a compensatory mechanism; Biomarker for obesity, T2DM and MetS. | ↑ | [55,56,59] |
| PGRN | Growth factor in epithelial cells, fibroblasts and adipocytes; Direct binding to TNFR inhibiting neutrophil activation; Contradictory results in the progression of insulin resistance and inflammation; PGRN expression may be upregulated by a compensatory mechanism; Change of PGRN negatively correlated with the improvement of current perception threshold after 6-month alpha-lipoic treatment in T2DM patients with peripheral neuropathy. | ↑↓ | [60,61,63,65,68] |
| Vaspin | Insulin-sensitizing and anti-inflammatory agent via a compensatory mechanism; Involvement in hypertension; Involvement in lipid metabolism; Biomarker for obesity, T2DM and NAFLD. | ↑↓ | [71,72,75,76] |
| Omentin-1 | Insulin sensitizing and anti-inflammatory agent; Biomarker for MetS, hypertension, T2DM and diabetic complications; Involvement in lipid metabolism | ↓ | [77,79,80,81] |
| Metrnl | Involvement in thermogenesis in brown/beige adipocytes; Insulin sensitizing and anti-inflammatory effects; Putative involvement in lipid metabolism. | ↑↓ | [85,86,89] |
| Hepatokines | |||
| Afamin | Association with adiposity, markers of MetS and NAFLD; Biomarker for the prevalence and incidence of T2DM and gestational diabetes; Biomarker for unstable atherosclerotic plaque; Involvement in HDL metabolism. | ↑ | [96,97,99,101] |
| RBP4 | Strong correlation with the markers of dyslipidemia including lipoprotein subfractions and ApoAI; Association with adiposity, insulin resistance, MetS and T2DM. | ↑↓ | [103,105,106,109] |
| Fetuin-A | Strong correlation with the markers of dyslipidemia including lipoprotein subfractions; Putative association to HDL proteome; Biomarker for obesity, MetS and T2DM; Biomarker for subclinical atherosclerosis. | ↑↓ | [109,113,115,117,123] |
| FGF21 | Insulin-sensitizing effects; Regulator of thermogenesis and lipolysis in the brown adipose tissue; FGF21 expression may be upregulated by a compensatory mechanism in obesity, T2DM and hypertension; Short term moderately intensive physical activity improved FGF21 levels in T2DM patients with peripheral neuropathy. | ↑↓ | [127,128,133,134,135] |
| Gut hormones | |||
| Obestatin | Anorexigenic and insulin-sensitizing effects; Involvement in the pathophysiology of obesity, insulin resistance and T2DM; Involvement in the regulation of lipid metabolism including VLDL and HDL subfractions, correlation with mean LDL size. | ↓ | [139,142,145] |
| Ghrelin | Orexigenic effects, stimulating gastric motility and hepatic glucose secretion and reducing insulin secretion; Inducing growth hormone and glucocorticoid secretion; Ghrelin expression is markedly decreased in obese individuals independently of gender. | ↓ | [147,152,203] |
| Myokines | |||
| Irisin | Insulin sensitizing hormone; Involvement in lipid metabolism; Biomarker for MetS, T2DM and cardiovascular diseases; Increased levels after physical activity; Decreased levels after weight loss due to bariatric surgery. | ↑↓ | [171,178,185,187] |
| Myostatin | Negative regulator of skeletal muscle growth; Levels are decreasing with age and exercise; Related to systemic inflammation and sarcopenic obesity. | ↑↓ | [191,193,195] |
| Follistatin | Promoting muscle mass and function; Potential therapeutic use for the treatment of muscle wasting in cachexic conditions; Involvement in beige and brown adipose tissue differentiation. | ↑↓ | [197,202] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lőrincz, H.; Somodi, S.; Ratku, B.; Harangi, M.; Paragh, G. Crucial Regulatory Role of Organokines in Relation to Metabolic Changes in Non-Diabetic Obesity. Metabolites 2023, 13, 270. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo13020270
Lőrincz H, Somodi S, Ratku B, Harangi M, Paragh G. Crucial Regulatory Role of Organokines in Relation to Metabolic Changes in Non-Diabetic Obesity. Metabolites. 2023; 13(2):270. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo13020270
Chicago/Turabian StyleLőrincz, Hajnalka, Sándor Somodi, Balázs Ratku, Mariann Harangi, and György Paragh. 2023. "Crucial Regulatory Role of Organokines in Relation to Metabolic Changes in Non-Diabetic Obesity" Metabolites 13, no. 2: 270. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo13020270