Metabolite Changes of Perna canaliculus Following a Laboratory Marine Heatwave Exposure: Insights from Metabolomic Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Husbandry and Haemolymph Collection
2.2. Metabolomics Sample Preparation, Analysis, and Data Processing
3. Results and Discussion
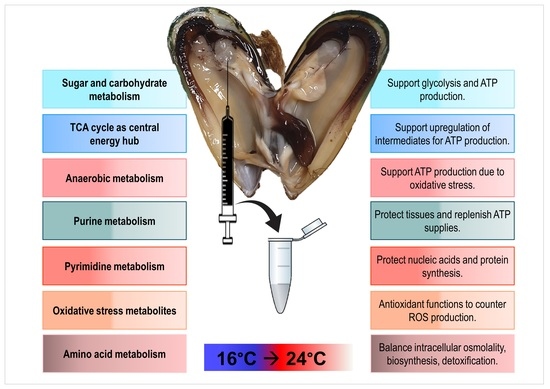
3.1. Sugar and Carbohydrate Metabolism to Support Energy Storage or Utilisation
3.2. TCA Cycle as Central Hub for Energy Metabolism and Redox Balance
3.3. Metabolites Countering Oxidative Stress
3.4. Anaerobic Energy Supply
3.5. Purine and Pyrimidine Metabolism
3.6. Amino Acid Metabolism
3.7. Understanding Mussel Metabolite Thermal Response in an Aquaculture Context
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, K.E.; Burrows, M.T.; Hobday, A.J.; King, N.G.; Moore, P.J.; Sen Gupta, A.; Thomsen, M.S.; Wernberg, T.; Smale, D.A. Biological impacts of marine heatwaves. Annu. Rev. Mar. Sci. 2023, 15, 119–145. [Google Scholar] [CrossRef]
- Stevens, C.L.; Spillman, C.M.; Behrens, E.; Broekhuizen, N.; Holland, P.; Matthews, Y.; Noll, B.; O’Callaghan, J.M.; Rampal, N.; Smith, R.O. Horizon scan on the benefits of ocean seasonal forecasting in a future of increasing marine heatwaves for Aotearoa New Zealand. Front. Clim. 2022, 92, 907919. [Google Scholar] [CrossRef]
- Behrens, E.; Rickard, G.; Rosier, S.; Williams, J.; Morgenstern, O.; Stone, D. Projections of future marine heatwaves for the oceans around New Zealand using New Zealand’s earth system model. Front. Clim. 2022, 19, 798287. [Google Scholar] [CrossRef]
- Ericson, J.; Venter, L.; Copedo, J.; Nguyen, V.; Alfaro, A.; Ragg, N. Chronic heat stress as a predisposing factor in summer mortality of mussels, Perna canaliculus. Aquaculture 2023, 564, 738986. [Google Scholar] [CrossRef]
- Stenton-Dozey, J.M.; Heath, P.; Ren, J.S.; Zamora, L.N. New Zealand aquaculture industry: Research, opportunities and constraints for integrative multitrophic farming. N. Z. J. Mar. Freshw. Res. 2021, 55, 265–285. [Google Scholar] [CrossRef]
- Fisheries New Zealand. The New Zealand Government Aquaculture Strategy: 2022 Implementation Plan; Ministry for Primary Industries: Wellington, New Zealand, 2022; ISBN 978-1-99-102653-8.
- Babcock, R.C.; Bustamante, R.H.; Fulton, E.A.; Fulton, D.J.; Haywood, M.D.; Hobday, A.J.; Kenyon, R.; Matear, R.J.; Plagányi, E.E.; Richardson, A.J. Severe continental-scale impacts of climate change are happening now: Extreme climate events impact marine habitat forming communities along 45% of Australia’s coast. Front. Mar. Sci. 2019, 6, 411. [Google Scholar] [CrossRef]
- Georgoulis, I.; Feidantsis, K.; Giantsis, I.A.; Kakale, A.; Bock, C.; Pörtner, H.O.; Sokolova, I.M.; Michaelidis, B. Heat hardening enhances mitochondrial potential for respiration and oxidative defence capacity in the mantle of thermally stressed Mytilus galloprovincialis. Sci. Rep. 2021, 11, 17098. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Gao, T.; Liu, X. Metabolomics adaptation of juvenile pacific abalone Haliotis discus hannai to heat stress. Sci. Rep. 2020, 10, 6353. [Google Scholar] [CrossRef] [Green Version]
- Delorme, N.J.; Venter, L.; Rolton, A.; Ericson, J.A. Integrating animal health and stress assessment tools using the green-lipped mussel Perna canaliculus as a case study. J. Shellfish Res. 2021, 40, 93–112. [Google Scholar] [CrossRef]
- Muznebin, F.; Alfaro, A.C.; Venter, L.; Young, T. Acute thermal stress and endotoxin exposure modulate metabolism and immunity in marine mussels (Perna canaliculus). J. Therm. Biol. 2022, 110, 103327. [Google Scholar] [CrossRef]
- Venter, L.; Alfaro, A.C.; Ragg, N.; Delorme, N.; Ericson, J.A. The effect of simulated marine heatwaves on green-lipped mussels, Perna canaliculus: A near-natural experimental approach. J. Therm. Biol. 2023. Under review. [Google Scholar]
- Alfaro, A.C.; Young, T. Showcasing metabolomic applications in aquaculture: A review. Rev. Aquac. 2018, 10, 135–152. [Google Scholar] [CrossRef]
- Young, T.; Alfaro, A.C. Metabolomic strategies for aquaculture research: A primer. Rev. Aquac. 2018, 10, 26–56. [Google Scholar] [CrossRef]
- Venter, L.; Young, T.; Alfaro, A.C.; Lindeque, J.Z. Establishing sampling confidence parameters: Effect of sampling and transport conditions on haemocyte and metabolite profiles of Greenshell mussels. Aquaculture 2021, 538, 736538. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Saratain, M. The Agilent Metabolomics Dynamic MRM Database and Method (5991-6482EN); Agilent Technologies Technical Overview; Agilent Technologies: Santa Clara, CA, USA, 2017. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Venter, L.; Mienie, L.; Vosloo, A.; Loots, D.; Jansen van Rensburg, P.; Lindeque, J. Effect of proline-enriched abalone feed on selected metabolite levels of slow-growing adult Haliotis midae. Aquac. Res. 2019, 50, 13978. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Goodacre, R. Multiblock principal component analysis: An efficient tool for analyzing metabolomics data which contain two influential factors. Metabolomics 2012, 8, 37–51. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, X.; Shi, Y.; Huang, W.; Sokolova, I.; Chang, X.; Chen, D.; Wei, S.; Khan, F.U.; Hu, M. Ocean acidification affects the bioenergetics of marine mussels as revealed by high-coverage quantitative metabolomics. Sci. Total Environ. 2023, 858, 160090. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferro, M.; Rocha, C.; Marques, J.C.; Gonçalves, A.M. Assessment of metal exposure (uranium and copper) in fatty acids and carbohydrate profiles of Calamoceras marsupus larvae (Trichoptera) and Alnus glutinosa leaf litter. Sci. Total Environ. 2022, 836, 155613. [Google Scholar] [CrossRef] [PubMed]
- Salway, J.G. Metabolism at a Glance; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Alam, Y.H.; Kim, R.; Jang, C. Metabolism and health impacts of dietary sugars. J. Lipid Atheroscler. 2022, 11, 20. [Google Scholar] [CrossRef]
- Matoo, O.B.; Lannig, G.; Bock, C.; Sokolova, I.M. Temperature but not ocean acidification affects energy metabolism and enzyme activities in the blue mussel, Mytilus edulis. Ecol. Evol. 2021, 11, 3366–3379. [Google Scholar] [CrossRef]
- Chen, H.; Solangi, G.S.; Zhao, C.; Yang, L.; Guo, J.; Wan, F.; Zhou, Z. Physiological metabolic responses of Ophraella communa to high temperature stress. Front. Physiol. 2019, 10, 1053. [Google Scholar] [CrossRef] [Green Version]
- Aguilera-Sáez, L.M.; Abreu, A.C.; Camacho-Rodríguez, J.; González-López, C.V.; del Carmen Cerón-García, M.a.; Fernández, I. NMR metabolomics as an effective tool to unravel the effect of light intensity and temperature on the composition of the marine microalgae Isochrysis galbana. J. Agric. Food Chem. 2019, 67, 3879–3889. [Google Scholar] [CrossRef]
- Chen, Y.-q.; Wang, J.; Liao, M.-l.; Li, X.-x.; Dong, Y.-w. Temperature adaptations of the thermophilic snail Echinolittorina malaccana: Insights from metabolomic analysis. J. Exp. Biol. 2021, 224, jeb238659. [Google Scholar] [CrossRef]
- Desai, T.A.; Rao, C.V. Regulation of arabinose and xylose metabolism in Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 1524–1532. [Google Scholar] [CrossRef] [Green Version]
- Seiboth, B.; Metz, B. Fungal arabinan and L-arabinose metabolism. Appl. Microbiol. Biotechnol. 2011, 89, 1665–1673. [Google Scholar] [CrossRef] [Green Version]
- Dunphy, B.; Ruggiero, K.; Zamora, L.; Ragg, N. Metabolomic analysis of heat-hardening in adult green-lipped mussel (Perna canaliculus): A key role for succinic acid and the GABAergic synapse pathway. J. Therm. Biol. 2018, 74, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Venter, L.; Alfaro, A.C.; Van Nguyen, T.; Lindeque, J.Z. Metabolite profiling of abalone (Haliotis iris) energy metabolism: A Chatham Islands case study. Metabolomics 2022, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-ketoglutarate: Physiological functions and applications. Biomol. Ther. 2016, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Legendre, F.; MacLean, A.; Appanna, V.; Appanna, V. Biochemical pathways to α-ketoglutarate, a multi-faceted metabolite. World J. Microbiol. Biotechnol. 2020, 36, 123. [Google Scholar] [CrossRef]
- Li, S.; Alfaro, A.C.; Nguyen, T.V.; Young, T.; Lulijwa, R. An integrated omics approach to investigate summer mortality of New Zealand Greenshell™ mussels. Metabolomics 2020, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Verlecar, X.; Jena, K.; Chainy, G. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem.-Biol. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef]
- Ericson, J.A.; Venter, L.; Welford, M.R.; Kumanan, K.; Alfaro, A.C.; Ragg, N.L. Effects of seawater temperature and acute Vibrio sp. challenge on the haemolymph immune and metabolic responses of adult mussels (Perna canaliculus). Fish Shellfish Immunol. 2022, 128, 664–675. [Google Scholar] [CrossRef]
- Noe, J.T.; Mitchell, R.A. Tricarboxylic acid cycle metabolites in the control of macrophage activation and effector phenotypes. J. Leukoc. Biol. 2019, 106, 359–367. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Alfaro, A.C.; Young, T.; Green, S.; Zarate, E.; Merien, F. Itaconic acid inhibits growth of a pathogenic marine Vibrio strain: A metabolomics approach. Sci. Rep. 2019, 9, 5937. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Wu, Y.; Li, J.; Peng, R.; Jiang, M.; Jiang, X.; Chen, S.; Lin, J. Effects of ammonia toxicity on the histopathology, detoxification, oxidative stress, and immune response of the cuttlefish Sepia pharaonis and the mitigation of γ-aminobutyric acid. Ecotoxicol. Environ. Saf. 2022, 232, 113256. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.W.; Li, Y.T.; Zhou, W.W.; Tian, L.X.; Li, Y.M.; Zeng, S.L.; Liu, Y.J. Effect of γ-aminobutyric acid supplementation on growth performance, endocrine hormone and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei, fed low fishmeal diet. Aquac. Nutr. 2017, 23, 54–62. [Google Scholar] [CrossRef]
- Zhang, C.; He, J.; Wang, X.; Yang, Y.; Huang, Q.; Qiao, F.; Shi, Q.; Qin, J.; Chen, L. Gamma-aminobutyric acid enhances hypoxia tolerance of juvenile Chinese mitten crab (Eriocheir sinensis) by regulating respiratory metabolism and alleviating neural excitotoxicity. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2022, 260, 109409. [Google Scholar] [CrossRef]
- Hui, T.Y.; Dong, Y.-w.; Han, G.-d.; Lau, S.L.; Cheng, M.C.; Meepoka, C.; Ganmanee, M.; Williams, G.A. Timing metabolic depression: Predicting thermal stress in extreme intertidal environments. Am. Nat. 2020, 196, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, B.J.; Watts, E.; Ragg, N.L. Identifying thermally-stressed adult green-lipped mussels (Perna canaliculus Gmelin, 1791) via metabolomic profiling. Am. Malacol. Bull. 2015, 33, 127–136. [Google Scholar] [CrossRef]
- Song, M.; Zhao, J.; Wen, H.-S.; Li, Y.; Li, J.-F.; Li, L.-M.; Tao, Y.-X. The impact of acute thermal stress on the metabolome of the black rockfish (Sebastes schlegelii). PLoS ONE 2019, 14, e0217133. [Google Scholar] [CrossRef] [Green Version]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef]
- Young, T.; Alfaro, A.C.; Villas-Bôas, S.G. Metabolic profiling of mussel larvae: Effect of handling and culture conditions. Aquac. Int. 2016, 24, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Ratnawati, S.E.; Kuuliala, L.; Walgraeve, C.; Demeestere, K.; Ragaert, P.; Devlieghere, F. The effect of high oxygen modified atmospheres on the quality degradation of packed live blue mussels (Mytilus edulis). LWT 2023, 177, 114537. [Google Scholar] [CrossRef]
- Dittrich, C.R.; Bennett, G.N.; San, K.Y. Metabolic engineering of the anaerobic central metabolic pathway in Escherichia coli for the simultaneous anaerobic production of isoamyl acetate and succinic acid. Biotechnol. Prog. 2009, 25, 1304–1309. [Google Scholar] [CrossRef]
- Zurburg, W.; De Zwaan, A. The role of amino acids in anaerobiosis and osmoregulation in bivalves. J. Exp. Zool. 1981, 215, 315–325. [Google Scholar] [CrossRef]
- Kluytmans, J.; Zandee, D.; Zurburg, W.; Pieters, H. The influence of seasonal changes on energy metabolism in Mytilus edulise (L.)—III. Anaerobic energy metabolism. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1980, 67, 307–315. [Google Scholar] [CrossRef]
- Sauve, A.A. NAD+ and vitamin B3: From metabolism to therapies. J. Pharmacol. Exp. Ther. 2008, 324, 883–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, J.; Kumar, R.; Yadav, S.K.; Jha, G. Nicotinic acid catabolism modulates bacterial mycophagy in Burkholderia gladioli strain NGJ1. Microbiol. Spectr. 2023, 11, e0445722. [Google Scholar] [CrossRef]
- Dong, X.; Yang, Z.; Liu, Z.; Wang, X.; Yu, H.; Peng, C.; Hou, X.; Lu, W.; Xing, Q.; Hu, J. Metabonomic analysis provides new insights into the response of Zhikong scallop (Chlamys farreri) to heat stress by improving energy metabolism and antioxidant capacity. Antioxidants 2022, 11, 1084. [Google Scholar] [CrossRef]
- Eymann, C.; Götze, S.; Bock, C.; Guderley, H.; Knoll, A.H.; Lannig, G.; Sokolova, I.M.; Aberhan, M.; Pörtner, H.-O. Thermal performance of the European flat oyster, Ostrea edulis (Linnaeus, 1758)—Explaining ecological findings under climate change. Mar. Biol. 2020, 167, 17. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, L.; Song, H.; Feng, J.; Zhou, C.; Yang, M.-J.; Shi, P.; Li, Y.-R.; Guo, Y.-J.; Li, H.-Z. Effect of heat and hypoxia stress on mitochondrion and energy metabolism in the gill of hard clam. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 266, 109556. [Google Scholar] [CrossRef]
- Young, T.; Kesarcodi-Watson, A.; Alfaro, A.C.; Merien, F.; Nguyen, T.V.; Mae, H.; Le, D.V.; Villas-Bôas, S. Differential expression of novel metabolic and immunological biomarkers in oysters challenged with a virulent strain of OsHV-1. Dev. Comp. Immunol. 2017, 73, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.-W.; Li, S.-Y.; Zhang, X.-L.; Chen, C.-Y.; Sun, W.-J.; Gu, Z.-Q.; Huang, J.; He, J.-Y.; Qi, P.-Z.; Guo, B.-Y. Morphological change and differential proteomics analysis of gill in Mytilus coruscus under starvation. Front. Physiol. 2023, 14, 426. [Google Scholar] [CrossRef]
- Dumas, T.; Bonnefille, B.; Gomez, E.; Boccard, J.; Castro, N.A.; Fenet, H.; Courant, F. Metabolomics approach reveals disruption of metabolic pathways in the marine bivalve Mytilus galloprovincialis exposed to a WWTP effluent extract. Sci. Total Environ. 2020, 712, 136551. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Fan, K.; Zhang, L.; Liu, Y.; Liu, P.-f. Metabolomics analysis of the effects of temperature on the growth and development of juvenile European seabass (Dicentrarchus labrax). Sci. Total Environ. 2021, 769, 145155. [Google Scholar] [CrossRef] [PubMed]
- Roznere, I.; Watters, G.T.; Wolfe, B.A.; Daly, M. Effects of relocation on metabolic profiles of freshwater mussels: Metabolomics as a tool for improving conservation techniques. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 919–926. [Google Scholar] [CrossRef]
- Georgoulis, I.; Bock, C.; Lannig, G.; Pörtner, H.-O.; Feidantsis, K.; Giantsis, I.A.; Sokolova, I.M.; Michaelidis, B. Metabolic remodeling caused by heat hardening in the Mediterranean mussel Mytilus galloprovincialis. J. Exp. Biol. 2022, 225, jeb244795. [Google Scholar] [CrossRef]
- Haskó, G.; Sitkovsky, M.V.; Szabo, C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol. Sci. 2004, 25, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, J.; Ma, H.; Lu, W.; Huang, J. Targeting pyrimidine metabolism in the era of precision cancer medicine. Front. Oncol. 2021, 11, 684961. [Google Scholar] [CrossRef]
- Truebano, M.; Burns, G.; Thorne, M.A.; Hillyard, G.; Peck, L.S.; Skibinski, D.O.; Clark, M.S. Transcriptional response to heat stress in the Antarctic bivalve Laternula elliptica. J. Exp. Mar. Biol. Ecol. 2010, 391, 65–72. [Google Scholar] [CrossRef]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Podrabsky, J.E.; Somero, G.N. Changes in gene expression associated with acclimation to constant temperatures and fluctuating daily temperatures in an annual killifish Austrofundulus limnaeus. J. Exp. Biol. 2004, 207, 2237–2254. [Google Scholar] [CrossRef] [Green Version]
- Ellis, R.P.; Spicer, J.I.; Byrne, J.J.; Sommer, U.; Viant, M.R.; White, D.A.; Widdicombe, S. 1H NMR metabolomics reveals contrasting response by male and female mussels exposed to reduced seawater pH, increased temperature, and a pathogen. Environ. Sci. Technol. 2014, 48, 7044–7052. [Google Scholar] [CrossRef]
- Meng, J.; Zhu, Q.; Zhang, L.; Li, C.; Li, L.; She, Z.; Huang, B.; Zhang, G. Genome and transcriptome analyses provide insight into the euryhaline adaptation mechanism of Crassostrea gigas. PLoS ONE 2013, 8, e58563. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Chen, W.; Lu, S.; Zhang, H.; Yin, L. Metabolic engineering of shikimic acid biosynthesis pathway for the production of shikimic acid and its branched products in microorganisms: Advances and prospects. Molecules 2022, 27, 4779. [Google Scholar] [CrossRef]
- Azizan, A.; Alfaro, A.C.; Young, T.; Venter, L. Beyond relaxed: Magnesium chloride anaesthesia alters the circulatory metabolome of a marine mollusc (Perna canaliculus). Metabolomics 2021, 17, 73. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Czumaj, A.; Szrok-Jurga, S.; Hebanowska, A.; Turyn, J.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The pathophysiological role of CoA. Int. J. Mol. Sci. 2020, 21, 9057. [Google Scholar] [CrossRef]
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de novo purine biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Siegle, M.R.; Taylor, E.B.; O’Connor, M.I. Prior heat accumulation reduces survival during subsequent experimental heat waves. J. Exp. Mar. Biol. Ecol. 2018, 501, 109–117. [Google Scholar] [CrossRef]
- Ericson, J.A.; Delorme, N.; Ragg, N.L.C. Heat Tolerance of Greenshell Mussels™ (Perna Canaliculus): Collated Research Findings & Implications for Mussel Farming; Cawthron Report No. 3914; Cawthron Institute: Nelson, New Zealand, 2023; 19p + appendices. [Google Scholar]
- Waller, D.L.; Cope, W.G. The status of mussel health assessment and a path forward. Freshw. Mollusk Biol. Conserv. 2019, 22, 26–42. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World Aquac. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Law, C.; Barr, N.; Gall, M.; Cummings, V.; Currie, K.; Murdoch, J.; Halliday, J.; Frost, E.; Stevens, C.; Plew, D. Futureproofing the Green-Lipped Mussel Aquaculture Industry against Ocean Acidification; National Institute for Water and Atmospheric Research: Auckland, New Zealand; University of Otago: Dunedin, New Zealand, 2020. [Google Scholar]
- Heasman, K.G.; Scott, N.; Ericson, J.A.; Taylor, D.I.; Buck, B.H. Extending New Zealand’s marine shellfish aquaculture into exposed environments–adapting to modern anthropogenic challenges. Front. Mar. Sci. 2020, 7, 565686. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizan, A.; Venter, L.; Jansen van Rensburg, P.J.; Ericson, J.A.; Ragg, N.L.C.; Alfaro, A.C. Metabolite Changes of Perna canaliculus Following a Laboratory Marine Heatwave Exposure: Insights from Metabolomic Analyses. Metabolites 2023, 13, 815. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo13070815
Azizan A, Venter L, Jansen van Rensburg PJ, Ericson JA, Ragg NLC, Alfaro AC. Metabolite Changes of Perna canaliculus Following a Laboratory Marine Heatwave Exposure: Insights from Metabolomic Analyses. Metabolites. 2023; 13(7):815. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo13070815
Chicago/Turabian StyleAzizan, Awanis, Leonie Venter, Peet J. Jansen van Rensburg, Jessica A. Ericson, Norman L. C. Ragg, and Andrea C. Alfaro. 2023. "Metabolite Changes of Perna canaliculus Following a Laboratory Marine Heatwave Exposure: Insights from Metabolomic Analyses" Metabolites 13, no. 7: 815. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo13070815