Hepatic Metabolic Derangements Triggered by Hyperthermia: An In Vitro Metabolomic Study
Abstract
:1. Introduction
2. Results
2.1. Hyperthermic Conditions Affect the Viability of PMH
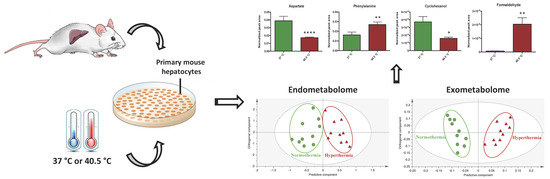
2.2. Hyperthermia Significantly Alters the Metabolic Profile of PMH
2.3. Discriminant Metabolites Identified
2.4. Hepatic Biochemical Pathways Affected by Hyperthermia
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation and Primary Culture of Mouse Hepatocytes
4.3. Cell Viability Assays
4.4. Collection, Preparation, and Analysis of Samples for Metabolomic Analysis
4.5. GC-MS Data Pre-Processing
4.6. Multivariate and Univariate Statistical Analysis
4.7. Identification of Discriminant Metabolites
4.8. Biochemical Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dao, C.K.; Nowinski, S.M.; Mills, E.M. The heat is on: Molecular mechanisms of drug-induced hyperthermia. Temperature 2014, 1, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, J.; Rummel, C.; Barth, S.W.; Gerstberger, R.; Hubschle, T. Molecular aspects of fever and hyperthermia. Neurol. Clin. 2006, 24, 421–439. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.D.; Martinez, J.P.; Barrueto, F., Jr. Drug-induced hyperthermic syndromes: Part I. Hyperthermia in overdose. Emerg. Med. Clin. N. Am. 2013, 31, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, N.; Dawson, A. The hot patient: Acute drug-induced hyperthermia. Aust. Prescr. 2019, 42, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; Tran, K.; de Haan, J.; de Man, R. Liver transplantation for non-exertional heat stroke-related acute liver failure. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Luca, L.E.; Nonner, D.; Saleh, O.; Hu, B.; Barrett, E.F.; Barrett, J.N. Cellular mechanisms of neuronal damage from hyperthermia. Prog. Brain Res. 2007, 162, 347–371. [Google Scholar] [CrossRef]
- Davis, B.C.; Tillman, H.; Chung, R.T.; Stravitz, R.T.; Reddy, R.; Fontana, R.J.; McGuire, B.; Davern, T.; Lee, W.M.; Acute Liver Failure Study Group. Heat stroke leading to acute liver injury & failure: A case series from the Acute Liver Failure Study Group. Liver Int. 2017, 37, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Garcin, J.M.; Bronstein, J.A.; Cremades, S.; Courbin, P.; Cointet, F. Acute liver failure is frequent during heat stroke. World J. Gastroenterol. 2008, 14, 158–159. [Google Scholar] [CrossRef]
- Skibba, J.L.; Powers, R.H.; Stadnicka, A.; Cullinane, D.W.; Almagro, U.A.; Kalbfleisch, J.H. Oxidative stress as a precursor to the irreversible hepatocellular injury caused by hyperthermia. Int. J. Hyperth. 1991, 7, 749–761. [Google Scholar] [CrossRef]
- Carvalho, F.; Remiao, F.; Soares, M.E.; Catarino, R.; Queiroz, G.; Bastos, M.L. d-Amphetamine-induced hepatotoxicity: Possible contribution of catecholamines and hyperthermia to the effect studied in isolated rat hepatocytes. Arch. Toxicol. 1997, 71, 429–436. [Google Scholar] [CrossRef]
- Carvalho, M.; Carvalho, F.; Bastos, M.L. Is hyperthermia the triggering factor for hepatotoxicity induced by 3,4-methylenedioxymethamphetamine (ecstasy)? An in vitro study using freshly isolated mouse hepatocytes. Arch. Toxicol. 2001, 74, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Katagiri, K.; Yamamoto, S.; Asanuma, S.; Usuda, M.; Kawahara, I.; Wakamatsu, K. Effect of hyperthermia on glutathione peroxidase and lipid peroxidative damage in liver. J. Therm. Biol. 1994, 19, 177–185. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central neural pathways for thermoregulation. Front. Biosci. 2011, 16, 74–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.A.; Djukovic, D. Overview of mass spectrometry-based metabolomics: Opportunities and challenges. Methods Mol. Biol. 2014, 1198, 3–12. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Halket, J.M.; Waterman, D.; Przyborowska, A.M.; Patel, R.K.P.; Fraser, P.D.; Bramley, P.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005, 56, 210–243. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Da Silva, D.D.; Silva, E.; Carmo, H. Combination effects of amphetamines under hyperthermia—The role played by oxidative stress. J. Appl. Toxicol. 2014, 34, 637–650. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A.; Haick, H. Hybrid volatolomics and disease detection. Angew. Chem. Int. Ed. Engl. 2015, 54, 11036–11048. [Google Scholar] [CrossRef] [PubMed]
- Reingruber, H.; Pontel, L.B. Formaldehyde metabolism and its impact on human health. Curr. Opin. Toxicol. 2018, 9, 28–34. [Google Scholar] [CrossRef]
- Gulec, M.; Gurel, A.; Armutcu, F. Vitamin E protects against oxidative damage caused by formaldehyde in the liver and plasma of rats. Mol. Cell. Biochem. 2006, 290, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Belanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Sousa Silva, M.; Gomes, R.A.; Ferreira, A.E.; Ponces Freire, A.; Cordeiro, C. The glyoxalase pathway: The first hundred years... and beyond. Biochem. J. 2013, 453, 1–15. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Hu, R.; Wang, Z.; Peng, Q.; Dong, X.; Zhang, X.; Zou, H.; Pu, Q.; Xue, B.; Wang, L. Metabolomics Profiling of Serum and Urine in Three Beef Cattle Breeds Revealed Different Levels of Tolerance to Heat Stress. J. Agric. Food Chem. 2018, 66, 6926–6935. [Google Scholar] [CrossRef]
- Fan, C.; Su, D.; Tian, H.; Li, X.; Li, Y.; Ran, L.; Hu, R.; Cheng, J. Liver metabolic perturbations of heat-stressed lactating dairy cows. Asian-Australas. J. Anim. Sci. 2018, 31, 1244–1251. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, C.; Hao, Y.; Gu, X.; Wang, H. Chronic Heat Stress Induces Acute Phase Responses and Serum Metabolome Changes in Finishing Pigs. Animals 2019, 9, 395. [Google Scholar] [CrossRef]
- Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.; Borner, C.; Bottger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, A.M.; Bastos, M.L.; Fernandes, E.; Carvalho, F.; Carvalho, M.; Guedes de Pinho, P. GC-MS metabolomics reveals disturbed metabolic pathways in primary mouse hepatocytes exposed to subtoxic levels of 3,4-methylenedioxymethamphetamine (MDMA). Arch. Toxicol. 2018, 92, 3307–3323. [Google Scholar] [CrossRef]
- Valente, M.J.; Araujo, A.M.; Silva, R.; Bastos Mde, L.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. 3,4-Methylenedioxypyrovalerone (MDPV): In vitro mechanisms of hepatotoxicity under normothermic and hyperthermic conditions. Arch. Toxicol. 2016, 90, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.M.; Moreira, N.; Lima, A.R.; Bastos, M.L.; Carvalho, F.; Carvalho, M.; de Pinho, P.G. Analysis of extracellular metabolome by HS-SPME/GC-MS: Optimization and application in a pilot study to evaluate galactosamine-induced hepatotoxicity. Toxicol. Lett. 2018, 295, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Hutschenreuther, A.; Kiontke, A.; Birkenmeier, G.; Birkemeyer, C. Comparison of extraction conditions and normalization approaches for cellular metabolomics of adherent growing cells with GC-MS. Anal. Methods 2012, 4. [Google Scholar] [CrossRef]
- Wheelock, A.M.; Wheelock, C.E. Trials and tribulations of ‘omics data analysis: Assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst. 2013, 9, 2589–2596. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Ferrarini, A.; Rey-Stolle, F.; Garcia, A.; Barbas, C. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 2015, 900, 21–35. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Berben, L.; Sereika, S.M.; Engberg, S. Effect size estimation: Methods and examples. Int. J. Nurs. Stud. 2012, 49, 1039–1047. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, A.M.; Enea, M.; Carvalho, F.; Bastos, M.d.L.; Carvalho, M.; Guedes de Pinho, P. Hepatic Metabolic Derangements Triggered by Hyperthermia: An In Vitro Metabolomic Study. Metabolites 2019, 9, 228. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo9100228
Araújo AM, Enea M, Carvalho F, Bastos MdL, Carvalho M, Guedes de Pinho P. Hepatic Metabolic Derangements Triggered by Hyperthermia: An In Vitro Metabolomic Study. Metabolites. 2019; 9(10):228. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo9100228
Chicago/Turabian StyleAraújo, Ana Margarida, Maria Enea, Félix Carvalho, Maria de Lourdes Bastos, Márcia Carvalho, and Paula Guedes de Pinho. 2019. "Hepatic Metabolic Derangements Triggered by Hyperthermia: An In Vitro Metabolomic Study" Metabolites 9, no. 10: 228. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo9100228