Does Cysteine Rule (CysR) Complete the CendR Principle? Increase in Affinity of Peptide Ligands for NRP-1 Through the Presence of N-Terminal Cysteine
Abstract
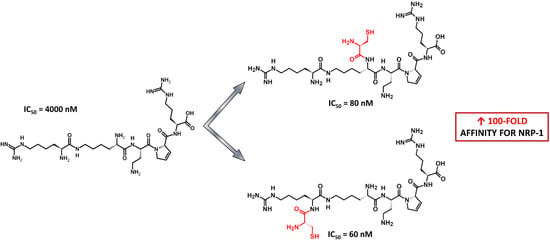
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Peptides Synthesis
2.3. Competitive NRP-1-Binding Assay
2.4. Degradation Assay in Human Plasma
2.5. Statistical Analysis
2.6. Ethics
3. Results and Discussion
3.1. Peptides Synthesis
3.2. VEGF-A165/NRP-1 Complex Inhibition Assay
3.3. Enzymatic Stability in Human Plasma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, Z.; Tessier-Lavigne, M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997, 90, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Goishi, K.; Davidson, A.J.; Mannix, R.; Zon, L.; Klagsbrun, M. Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc. Natl. Acad. Sci. USA 2002, 99, 10470–10475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staton, C.A.; Kumar, I.; Reed, M.W.; Brown, N.J. Neuropilins in physiological and pathological angiogenesis. J. Pathol. 2007, 212, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Zachary, I.C. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem. Soc. Trans. 2011, 39, 1583–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamluk, R.; Gechtman, Z.; Kutcher, M.E.; Gasiunas, N.; Gallagher, J.; Klagsbrun, M. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J. Biol. Chem. 2002, 277, 24818–24825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Guo, H.F.; Vander Kooi, C.W. Neuropilin functions as an essential cell surface receptor. J. Biol. Chem. 2015, 290, 29120–29126. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.Q.; Lee, P.; Lin, H.; Soker, S.; Klagsbrun, M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000, 14, 2532–2539. [Google Scholar] [CrossRef] [Green Version]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, T.; Tokunaga, T.; Hatanaka, H.; Kijima, H.; Yamazaki, H.; Abe, Y.; Osamura, Y.; Inoue, H.; Ueyama, Y.; Nakamura, M. Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer 2002, 95, 2196–2201. [Google Scholar] [CrossRef]
- Bagri, A.; Tessier-Lavigne, M.; Watts, R.J. Neuropilins in tumor biology. Clin. Cancer Res. 2009, 15, 1860–1864. [Google Scholar] [CrossRef] [Green Version]
- Prud’homme, G.J.; Glinka, Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2012, 9, 921–939. [Google Scholar] [CrossRef] [Green Version]
- Wild, J.R.L.; Staton, C.A.; Chapple, K.; Corfe, B.M. Neuropilins: expression and roles in the epithelium. Int. J. Exp. Pathol. 2012, 93, 81–103. [Google Scholar] [CrossRef]
- Keyt, B.A.; Berleau, L.T.; Nguyen, H.V.; Chen, H.; Heinsohn, H.; Vandeln, R.; Ferrara, N. The carboxyl-terminal domain (111-165) of vascular endothelial growth factor is critical for its mitogenic potency. J. Biol. Chem. 1996, 271, 7788–7795. [Google Scholar] [CrossRef] [Green Version]
- Soker, S.; Gollamudi-Payne, S.; Fidder, H.; Charmahelli, H.; Klagsbrun, M. Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation by a peptide corresponding to the exon 7-encoded domain of VEGF165. J. Biol. Chem. 1997, 272, 31582–31588. [Google Scholar] [CrossRef] [Green Version]
- Fairbrother, W.J.; Chample, M.A.; Christinger, H.W.; Keyt, B.A.; Starovasnik, M.A. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure 1998, 6, 637–648. [Google Scholar] [CrossRef]
- Parker, M.W.; Xu, P.; Li, X.; Vander Kooi, C.W. Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J. Biol. Chem. 2012, 14, 11082–11089. [Google Scholar] [CrossRef] [Green Version]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef] [Green Version]
- Binétruy-Tournaire, R.; Demangel, C.; Malavaud, B.; Vassy, R.; Rouyre, S.; Kraemer, M.; Plouët, J.; Derbin, C.; Perret, G.; Mazié, J.C. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000, 19, 1525–1533. [Google Scholar] [CrossRef] [Green Version]
- Perret, G.Y.; Starzec, A.; Hauet, N.; Vergote, J.; Le Pecheur, M.; Vassy, R.; Léger, G.; Verbeke, K.A.; Bormans, G.; Nicolas, P.; et al. In vitro evaluation and biodistribution of a 99mTc-labeled anti-VEGF peptide targeting neuropilin-1. Nucl. Med. Biol. 2004, 31, 575–581. [Google Scholar] [CrossRef]
- Starzec, A.; Vassy, R.; Martin, A.; Lecouvey, M.; Di Benedetto, M.; Crépin, M.; Perret, G.Y. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006, 79, 2370–2381. [Google Scholar] [CrossRef]
- Fedorczyk, B.; Lipiński, P.F.J.; Tymecka, D.; Puszko, A.K.; Wilenska, B.; Perret, G.Y.; Misicka, A. Conformational latitude - activity relationship of KPPR tetrapeptide analogues toward their ability to inhibit binding of vascular endothelial growth factor 165 to neuropilin-1. J. Pept. Sci. 2017, 23, 445–454. [Google Scholar] [CrossRef]
- Von Wronski, M.A.; Raju, N.; Pillai, R.; Bogdan, N.J.; Marinelli, E.R.; Nanjappan, P.; Ramalingam, K.; Arunachalam, T.; Eaton, S.; Linder, K.E.; et al. Tuftsin binds neuropilin-1 through a sequence similar to that encoded by exon 8 of vascular endothelial growth factor. J. Biol. Chem. 2006, 281, 5702–5710. [Google Scholar] [CrossRef] [Green Version]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef] [Green Version]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.M.; Blot, V.; Janvier, S.; Arnulf, B.; van Endert, P.M.; Heveker, N.; Pique, C.; et al. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef] [Green Version]
- Lambert, S.; Bouttier, M.; Vassy, R.; Seigneuret, M.; Petrow-Sadowski, C.; Janvier, S.; Heveker, N.; Ruscetti, F.W.; Perret, G.; Jones, K.S.; et al. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood 2009, 113, 5176–5185. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.B.; Zhang, H.; Zhang, J.P.; Li, Y.; Zhao, B.; Feng, G.K.; Du, Y.; Xiong, D.; Zhong, Q.; Liu, W.L.; et al. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat. Commun. 2015, 6, 6240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Pontén, A.; Aase, K.; Karlsson, L.; Abramsson, A.; Uutela, M.; Bäckström, G.; Hellström, M.; Boström, H.; Li, H.; et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat. Cell. Biol. 2000, 2, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Q.; Borriello, L.; Allain, B.; Pavoni, S.; Lopez, N.; Hermine, O.; Garbay, C.; Raynaud, F.; Lepelletier, Y.; Demange, L. New Peptides Structurally Related to VEGF-A165 Exon-7 and -8 Encoded Domains Antagonize Its Binding to NRP-1 and VEGF-R1. Int. J. Pept. Res. Ther. 2015, 21, 117–124. [Google Scholar] [CrossRef]
- Wennmohs, F.; Staemmler, V.; Schindler, M. Theoretical investigation of weak hydrogen bonds to sulfur. J. Chem. Phys. 2003, 119, 3208–3218. [Google Scholar] [CrossRef]
- Biswal, H.S.; Shirhatti, P.R.; Wategaonkar, S. O-H···O versus O-H···S hydrogen bonding. 2. Alcohols and thiols as hydrogen bond acceptors. J. Phys. Chem. A 2010, 114, 6944–6955. [Google Scholar] [CrossRef] [PubMed]
- Ringer, A.L.; Senenko, A.; Sherrill, C.D. Models of S/pi Interactions in Protein Structures: Comparison of the H2S Benzene Complex With PDB Data. Protein Sci. 2007, 16, 2216–2223. [Google Scholar] [CrossRef] [Green Version]
- Daeffler, K.N.-M.; Lester, H.A.; Dougherty, D.A. Functionally Important Aromatic-Aromatic and Sulfur-π Interactions in the D2 Dopamine Receptor. J. Am. Chem. Soc. 2012, 134, 14890–14896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drobecq, H.; Boll, E.; Sénéchal, M.; Desmet, R.; Saliou, J.M.; Lacapère, J.J.; Mougel, A.; Vicogne, J.; Melnyk, O. A Central Cysteine Residue Is Essential for the Thermal Stability and Function of SUMO-1 Protein and SUMO-1 Peptide-Protein Conjugates. Bioconjug. Chem. 2016, 27, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Putzu, M.; Gräter, F.; Elstner, M.; Kubař, T. On the Mechanism of Spontaneous Thiol-Disulfide Exchange in Proteins. Phys. Chem. Chem. Phys. 2018, 20, 16222–16230. [Google Scholar] [CrossRef]
- Passam, F.J.; Chiu, J. Allosteric Disulphide Bonds as Reversible Mechano-Sensitive Switches That Control Protein Functions in the Vasculature. Biophys. Rev. 2019, 11, 419–430. [Google Scholar] [CrossRef]
- Tymecka, D.; Puszko, A.K.; Lipiński, P.F.; Fedorczyk, B.; Wileńska, B.; Sura, K.; Perret, G.Y.; Misicka, A. Branched Pentapeptides as Potent Inhibitors of the Vascular Endothelial Growth Factor 165 Binding to Neuropilin-1: Design, Synthesis and Biological Activity. Eur. J. Med. Chem. 2018, 158, 453–462. [Google Scholar] [CrossRef]
- Puszko, A.K.; Sosnowski, P.; Tymecka, D.; Raynaud, F.; Hermine, O.; Lepelletier, Y.; Misicka, A. Neuropilin-1 Peptide-Like Ligands With Proline Mimetics, Tested Using the Improved Chemiluminescence Affinity Detection Method. Med. Chem. Comm. 2019, 10, 332–340. [Google Scholar] [CrossRef]
- Allain, B.; Jarray, R.; Borriello, L.; Leforban, B.; Dufour, S.; Liu, W.Q.; Pamonsinlapatham, P.; Bianco, S.; Larghero, J.; Hadj-Slimane, R.; et al. Neuropilin-1 Regulates a New VEGF-induced Gene, Phactr-1, Which Controls Tubulogenesis and Modulates Lamellipodial Dynamics in Human Endothelial Cells. Cell Signal. 2012, 24, 214–223. [Google Scholar] [CrossRef]
- Borriello, L.; Montès, M.; Lepelletier, Y.; Leforban, B.; Liu, W.Q.; Demange, L.; Delhomme, B.; Pavoni, S.; Jarray, R.; Boucher, J.L.; et al. Structure-based Discovery of a Small Non-Peptidic Neuropilins Antagonist Exerting in Vitro and in Vivo Anti-Tumor Activity on Breast Cancer Model. Cancer Lett. 2014, 349, 120–127. [Google Scholar] [CrossRef]
- Puszko, A.K.; Sosnowski, P.; Pułka-Ziach, K.; Hermine, O.; Hopfgartner, G.; Lepelletier, Y.; Misicka, A. Urea Moiety as Amide Bond Mimetic in Peptide-Like Inhibitors of VEGF-A165/NRP-1 Complex. Bioorg. Med. Chem. Lett. 2019, 29, 2493–2497. [Google Scholar] [CrossRef]
- Carpino, L.A.; Han, G. 9-Fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 1972, 37, 3404–3409. [Google Scholar] [CrossRef]
- Carpino, L.A. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. Am. Chem. Soc. 1993, 115, 4397–4398. [Google Scholar] [CrossRef]
- Starzec, A.; Ladam, P.; Vassy, R.; Badache, S.; Bouchemal, N.; Navaza, A.; du Penhoat, C.H.; Perret, G.Y. Structure-function Analysis of the Antiangiogenic ATWLPPR Peptide Inhibiting VEGF(165) Binding to neuropilin-1 and Molecular Dynamics Simulations of the ATWLPPR/neuropilin-1 Complex. Peptides 2007, 28, 2397–2402. [Google Scholar] [CrossRef]
- Vander Kooi, C.W.; Jusino, M.A.; Perman, B.; Neau, D.B.; Bellamy, H.D.; Leahy, D.J. Structural Basis for Ligand and Heparin Binding to Neuropilin B Domains. PNAS 2007, 104, 6152–6157. [Google Scholar] [CrossRef] [Green Version]
- Kamarulzaman, E.E.; Vanderesse, R.; Gazzali, A.M.; Barberi-Heyob, M.; Boura, C.; Frochot, C.; Shawkataly, O.; Aubry, A.; Wahab, H.A. Molecular Modelling, Synthesis and Biological Evaluation of Peptide Inhibitors as Anti-Angiogenic Agent Targeting neuropilin-1 for Anticancer Application. J. Biomol. Struct. Dyn. 2017, 35, 26–45. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, N.D. Peptidase Specificity From the Substrate Cleavage Collection in the MEROPS Database and a Tool to Measure Cleavage Site Conservation. Biochimie 2016, 122, 5–30. [Google Scholar] [CrossRef] [Green Version]
- De Vriese, C.; Gregoire, F.; Lema-Kisoka, R.; Waelbroeck, M.; Robberecht, P.; Delporte, C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology 2004, 145, 4997–5005. [Google Scholar] [CrossRef] [Green Version]
- Mansfeld, F.M.; Toth, I. Synthesis and Plasma Stability of Disulfide-Bridged Cyclic Endomorphin-1 Derivatives. Int. J. Org. Chem. 2012, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.; Vogel, H.J. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef] [Green Version]
- Janecka, A.; Kruszynski, R.; Fichna, J.; Kosson, P.; Janecki, T. Enzymatic degradation studies of endomorphin-2 and its analogs containing N-methylated amino acids. Peptides 2006, 27, 131–135. [Google Scholar] [CrossRef]



| Compound | Sequence | logIC50 | IC50 (µM) |
|---|---|---|---|
| PP 1 | H-Lys(hArg)-Dab-Dhp-Arg-OH | −5.54 ± 0.04 | 4.3 2 |
| 1 | H-Cys-Asp-Lys(hArg)-Dab-Dhp-Arg-OH | −5.60 ± 0.03 | 2.5 |
| 2 | H-Lys(Cys-Asp-hArg)-Dab-Dhp-Arg-OH | −5.70 ± 0.05 | 2.0 |
| 3 | H-Cys-Lys(hArg)-Dab-Dhp-Arg-OH | −7.079 ± 0.1316 | 0.08 |
| 4 | H-Lys(Cys-hArg)-Dab-Dhp-Arg-OH | −7.194 ± 0.0939 | 0.06 |
| Compound | Sequence | logIC50 | IC50 (µM) | Reference Peptide (RP) | RP IC50 (µM) |
|---|---|---|---|---|---|
| 5 | CLPPR | –4.94 ± 0.08 | 11.4 | A7R 1 | 80 (19,38) |
| 6 | CTKPR | –5.03 ± 0.08 | 9.3 | Tuftsin 2 | ≥100 (23) 3 |
| 7 | CKPRR | –6.727 ± 0.762 | 0.19 | DKPRR | >>100 (29,41) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puszko, A.K.; Sosnowski, P.; Raynaud, F.; Hermine, O.; Hopfgartner, G.; Lepelletier, Y.; Misicka, A. Does Cysteine Rule (CysR) Complete the CendR Principle? Increase in Affinity of Peptide Ligands for NRP-1 Through the Presence of N-Terminal Cysteine. Biomolecules 2020, 10, 448. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030448
Puszko AK, Sosnowski P, Raynaud F, Hermine O, Hopfgartner G, Lepelletier Y, Misicka A. Does Cysteine Rule (CysR) Complete the CendR Principle? Increase in Affinity of Peptide Ligands for NRP-1 Through the Presence of N-Terminal Cysteine. Biomolecules. 2020; 10(3):448. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030448
Chicago/Turabian StylePuszko, Anna K., Piotr Sosnowski, Françoise Raynaud, Olivier Hermine, Gérard Hopfgartner, Yves Lepelletier, and Aleksandra Misicka. 2020. "Does Cysteine Rule (CysR) Complete the CendR Principle? Increase in Affinity of Peptide Ligands for NRP-1 Through the Presence of N-Terminal Cysteine" Biomolecules 10, no. 3: 448. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030448