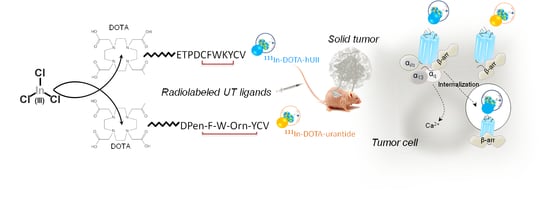
Development of Novel 111-In-Labelled DOTA Urotensin II Analogues for Targeting the UT Receptor Overexpressed in Solid Tumours
Abstract
:1. Introduction
2. Materials and Methods
2.1. Products and Reagents
2.2. Synthesis of Human UII and Urantide Conjugated to DOTA
2.2.1. Reagents and Materials
2.2.2. Resin Preparation
2.2.3. Synthesis on Tribute Synthetiser
2.3. Radiolabeling and Stability
Optimisation of the Radiolabelling
2.4. Serum Stability
2.5. Cell Lines Culture and Transfections
2.6. Binding Assay
2.7. Calcium Assay
2.8. Internalisation Assay
2.9. Cell Migration Assay
2.10. Animal Models
2.11. Immunochemistry
2.12. In Vivo Assay
2.13. Statistical Analyses
3. Results and Discussion
3.1. Synthesis and Radiolabelling of DOTA-hUII and DOTA-Urantide
3.2. Recognition and Activation of UT Receptor by DOTA-hUII and DOTA-urantide
3.3. Internalisation of UT Evoked by Urotensinergic DOTA Analogues
3.4. UT Expression and Function in DLD-1 and A549 Tumour Cell Lines
3.5. Biodistribution of 111In-DOTA-hUII and 111In-DOTA-Urantide
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Charron, C.L.; Hickey, J.L.; Nsiama, T.K.; Cruickshank, D.R.; Turnbull, W.L.; Luyt, L.G. Molecular imaging probes derived from natural peptides. Nat. Prod. Rep. 2016, 33, 761–800. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S.M.; Maecke, H.R. Radiometallo-labeled peptides in tumor diagnosis and targeted radionuclide therapy. In Advances in Inorganic Chemistry, van Eldik, R.; Hubbard, C.D., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 341–396. [Google Scholar] [CrossRef]
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillyar, C.R.T.; Cornelissen, B.; Vallis, K.A. Uptake, internalization and nuclear translocation of radioimmunotherapeutic agents. Ther. Deliv. 2014, 5, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Pae, J.; Pooga, M. Peptide-mediated delivery: An overview of pathways for efficient internalization. Ther. Deliv. 2014, 5, 1203–1222. [Google Scholar] [CrossRef] [PubMed]
- Mariani, G.; Erba, P.A.; Signore, A. Receptor-mediated tumor targeting with radiolabeled peptides: There is more to it than somatostatin analogs. J. Nucl. Med. 2006, 47, 1904–1907. [Google Scholar] [PubMed]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S.M.; Maecke, H.R. Radiolabelled peptides in medical imaging. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Woodhead Publishing Limited: Cambridge, UK, 2018; pp. 431–483. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schär, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Brabander, T.; Kwekkeboom, D.J.; Feelders, R.A.; Brouwers, A.H.; Teunissen, J.J.M. Nuclear medicine imaging of neuroendocrine tumors. Front. Horm. Res. 2015, 44, 73–87. [Google Scholar] [CrossRef]
- Kjaer, A.; Knigge, U. Use of radioactive substances in diagnosis and treatment of neuroendocrine tumors. Scand. J. Gastroenterol. 2015, 50, 740–747. [Google Scholar] [CrossRef]
- Brabander, T.; Teunissen, J.J.M.; Van Eijck, C.H.J.; Franssen, G.J.H.; Feelders, R.A.; de Herder, W.W.; Kwekkeboom, D.J. Peptide receptor radionuclide therapy of neuroendocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 103–114. [Google Scholar] [CrossRef]
- Deppen, S.A.; Blume, J.; Bobbey, A.J.; Shah, C.; Graham, M.M.; Lee, P.; Delbeke, D.; Walker, R.C. 68Ga-DOTATATE compared with 111In-DTPA-Octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: A systematic review and meta-analysis. J. Nucl. Med. 2016, 57, 872–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinfelder, J.; Kuwert, T.; Beck, M.; Sanders, J.C.; Ritt, P.; Schmidkonz, C.; Hennig, P.; Prante, O.; Uder, M.; Wullich, B.; et al. First experience with Spect/ct using a 99mtc-labeled inhibitor for prostate-specific membrane antigen in patients with biochemical recurrence of prostate cancer. Clin. Nucl. Med. 2017, 42, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.M.; Kern, A.M.; Maresca, K.P.; Marquis, J.C.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 123I-MIP-1072, a small-molecule inhibitor of prostate-specific membrane antigen, is effective at monitoring tumor response to taxane therapy. J. Nucl. Med. 2011, 52, 1087–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, J.; Afridi, A.; Vatsia, S.; Joshi, G.; Kaplan, S.A.; Smith, N.L.; Khan, S.A. The molecular biology of prostate cancer: Current understanding and clinical implications. Prostate Cancer Prostatic. Dis. 2017, 21, 22–36. [Google Scholar] [CrossRef]
- Ananias, H.J.K.; Yu, Z.; Dierckx, R.A.; van der Wiele, C.; Helfrich, W.; Wang, F.; Yan, Y.; Chen, X.; de Jong, I.J.; Elsinga, P.H. 99mTechnetium-HYNIC(tricine/TPPTS)-Aca-Bombesin(7–14) as a targeted imaging agent with microSPECT in a PC-3 prostate cancer xenograft model. Mol. Pharm. 2011, 8, 1165–1173. [Google Scholar] [CrossRef]
- Aranda-Lara, L.; Ferro-Flores, G.; Azorín-Vega, E.; de Ramírez, F.M.; Jiménez-Mancilla, N.; Ocampo-García, B.; Santos-Cuevas, C.; Isaac-Olivé, K. Synthesis and evaluation of Lys1(α,γ-Folate)Lys3(177Lu-DOTA)-Bombesin(1-14) as a potential theranostic radiopharmaceutical for breast cancer. Appl. Radiat. Isot. 2016, 107, 214–219. [Google Scholar] [CrossRef]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled Peptides: Valuable Tools for the Detection and Treatment of Cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [Green Version]
- Kwekkeboom, D.J.; Kam, B.L.; van Essen, M.; Teunissen, J.J.M.; van Eijck, C.H.J.; Valkema, R.; de Jong, M.; de Herder, W.W.; Krenning, E.P. Somatostatin receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2010, 17, R53–R73. [Google Scholar] [CrossRef] [Green Version]
- Weiss, I.D.; Jacobson, O. Molecular imaging of chemokine receptor CXCR4. Theranostics 2013, 3, 76–84. [Google Scholar] [CrossRef]
- Ilan, E.; Sandström, M.; Wassberg, C.; Sundin, A.; Garske–Román, U.; Eriksson, B.; Granberg, D.; Lubberink, M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J. Nucl. Med. 2015, 56, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Ljungberg, M.; Celler, A.; Konijnenberg, M.W.; Eckerman, K.F.; Dewaraja, Y.K.; Sjögreen-Gleisner, K. Joint EANM/MIRD Guidelines for Quantitative 177Lu SPECT Applied for Dosimetry of Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 151–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljungberg, M.; Pretorius, P.H. SPECT/CT: An update on technological developments and clinical applications. Br. J. Radiol. 2018, 91, 20160402. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]Octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tostivint, H.; Joly, L.; Lihrmann, I.; Parmentier, C.; Lebon, A.; Morisson, M.; Calas, A.; Ekker, M.; Vaudry, H. Comparative genomics provides evidence for close evolutionary relationships between the urotensin II and somatostatin gene families. Proc. Natl. Acad. Sci. USA 2006, 103, 2237–2242. [Google Scholar] [CrossRef] [Green Version]
- Castel, H.; Diallo, M.; Chatenet, D.; Leprince, J.; Desrues, L.; Schouft, M.T.; Fontaine, M.; Dubessy, C.; Lihrmann, I.; Scalbert, E.; et al. Biochemical and functional characterization of high-affinity urotensin II receptors in rat cortical astrocytes. J. Neurochem. 2006, 9, 582–595. [Google Scholar] [CrossRef]
- Desrues, L.; Lefebvre, T.; Diallo, M.; Gandolfo, P.; Leprince, J.; Chatenet, D.; Vaudry, H.; Tonon, M.C.; Castel, H. Effect of GABAA receptor activation on UT-coupled signaling pathways in rat cortical astrocytes. Peptides 2008, 29, 727–734. [Google Scholar] [CrossRef]
- Jarry, M.; Diallo, M.; Lecointre, C.; Desrues, L.; Tokay, T.; Chatenet, D.; Leprince, J.; Rossi, O.; Vaudry, H.; Tonon, M.C.; et al. The vasoactive peptides urotensin II and urotensin II-related peptide regulate astrocyte activity through common and distinct mechanisms: Involvement in cell proliferation. Biochem. J. 2010, 428, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Camarda, V.; Rizzi, A.; Calò, G.; Gendron, G.; Perron, S.I.; Kostenis, E.; Zamboni, P.; Mascoli, F.; Regoli, D. Effects of human urotensin II in isolated vessels of various species; comparison with other vasoactive agents. Naunyn Schmiedebergs Arch. Pharmacol. 2002, 365, 141–149. [Google Scholar] [CrossRef]
- Albertin, G.; Casale, V.; Ziolkowska, A.; Spinazzi, R.; Malendowicz, L.K.; Rossi, G.P.; Nussdorfer, G.G. Urotensin-II and UII-receptor expression and function in the rat adrenal cortex. Int. J. Mol. Med. 2006, 17, 1111–1115. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, R.A.; Egido, E.M.; Hernández, R.; Marco, J. Characterization of the insulinostatic effect of urotensin II: A study in the perfused rat pancreas. Regul. Pept. 2009, 153, 37–42. [Google Scholar] [CrossRef]
- Castel, H.; Desrues, L.; Joubert, J.E.; Tonon, M.C.; Prézeau, L.; Chabbert, M.; Morin, F.; Gandolfo, P. The G protein-coupled receptor UT of the neuropeptide urotensin II displays structural and functional chemokine features. Front. Endocrinol. 2017, 8, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakala, R. Role of urotensin II in atherosclerotic cardiovascular diseases. Cardiovasc. Revasc. Med. 2008, 9, 166–178. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Genest, J., Jr.; Barrette, P.O.; Hafiane, A.; Behm, D.J.; D’Orleans-Juste, P.; Schwertani, A.G. Genetic and pharmacological manipulation of urotensin II ameliorate the metabolic and atherosclerosis sequalae in mice. Atherioscler. Thromb. Vasc. Biol. 2012, 32, 1809–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segain, J.P.; Rolli-Derkinderen, M.; Gervois, N.; de la Blétière, D.R.; Loirand, G.; Pacaud, P. Urotensin II is a New Chemotactic Factor for UT Receptor-Expressing Monocytes. J. Immunol. 2007, 179, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Clavier, T.; Besnier, E.; Maucotel, J.; Arabo, A.; Desrues, L.; Amki, M.E.; Perzo, N.; Richard, V.; Tamion, F.; Gandolfo, P.; et al. Urantide improves cardiac function, modulates systemic cytokine response and increases survival in a murine model of endotoxic shock. Shock 2019, 1. [Google Scholar] [CrossRef]
- Spinazzi, R.; Albertin, G.; Nico, B.; Guidolin, D.; Di Liddo, R.; Rossi, G.P.; Ribatti, D.; Nussdorfer, G.G. Urotensin-II and its receptor (UT-R) are expressed in rat brain endothelial cells, and urotensin-II via UT-R stimulates angiogenesis in vivo and in vitro. Int. J. Mol. Med. 2006, 18, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Brulé, C.; Perzo, N.; Joubert, J.E.; Sainsily, X.; Leduc, R.; Castel, H.; Prézeau, L. Biased signaling regulates the pleiotropic effects of the urotensin II receptor to modulate its cellular behaviors. FASEB J. 2014, 28, 5148–5162. [Google Scholar] [CrossRef]
- Takahashi, K.; Totsune, K.; Murakami, O.; Arihara, Z.; Noshiro, T.; Hayashi, Y.; Shibahara, S. Expression of urotensin II and its receptor in adrenal tumors and stimulation of proliferation of cultured tumor cells by urotensin II. Peptides 2003, 24, 301–306. [Google Scholar] [CrossRef]
- Balakan, O.; Kalender, M.E.; Suner, A.; Cengiz, B.; Oztuzcu, S.; Bayraktar, R.; Borazan, E.; Babacan, T.; Camci, C. The relationship between urotensin II and its receptor and the clinicopathological parameters of breast cancer. Med. Sci. Monit. 2014, 20, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Kristof, A.S.; You, Z.; Han, Y.S.; Giaid, A. Protein expression of urotensin II, urotensin-related peptide and their receptor in the lungs of patients with lymphangioleiomyomatosis. Peptides 2010, 31, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Yu, X.; Xue, X.; Liu, H.; Wang, M.; Li, Y.; Wang, X.; Ding, H. Urotensin II receptor as a potential biomarker for the prognosis of hepatocellular carcinoma patients. Oncol. Lett. 2017, 14, 2749–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federico, A.; Zappavigna, S.; Romano, M.; Grieco, P.; Luce, A.; Marra, M.; Gravina, A.G.; Stiuso, P.; D’Armiento, F.P.; Vitale, G.; et al. Urotensin-II receptor is over-expressed in colon cancer cell lines and in colon carcinoma in humans. Eur. J. Clin. Invest. 2014, 44, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Song, Z.; Zhou, C.H.; Xing, S.H.; Pei, D.S.; Zheng, J.N. Expression of urotensin II and its receptor in human lung adenocarcinoma A549 cells and the effect of urotensin II on lung adenocarcinoma growth in vitro and in vivo. Oncol. Rep. 2010, 24, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Lecointre, C.; Desrues, L.; Joubert, J.E.; Perzo, N.; Guichet, P.O.; Le Joncour, V.; Brulé, C.; Chabbert, M.; Leduc, R.; Prézeau, L.; et al. Signaling switch of the urotensin II vasosactive peptide GPCR: Prototypic chemotaxic mechanism in glioma. Oncogene 2015, 34, 5080–5094. [Google Scholar] [CrossRef]
- Coly, P.M.; Perzo, N.; Le Joncour, V.L.; Lecointre, C.; Schouft, M.T.; Desrues, L.; Tonon, M.C.; Wurtz, O.; Gandolfo, P.; Castel, H.; et al. Chemotactic G protein-coupled receptors control cell migration by repressing autophagosome biogenesis. Autophagy 2016, 12, 2344–2362. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.H.; Wan, Y.Y.; Chu, X.H.; Song, Z.; Xing, S.H.; Wu, Y.Q.; Yin, X.X. Urotensin II contributes to the formation of lung adenocarcinoma inflammatory microenvironment through the NF-κB pathway in tumor-bearing nude mice. Oncol. Lett. 2012, 4, 1259–1263. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Park, B.K.; Oh, K.S.; Yi, K.Y.; Lim, C.J.; Seo, H.W.; Lee, B.H. A urotensin II receptor antagonist, KR36676, decreases vascular remodeling and inflammation in experimental pulmonary hypertension. Int. Immunopharmacol. 2016, 40, 196–202. [Google Scholar] [CrossRef]
- Qi, J.; Minor, L.K.; Smith, C.; Hu, B.; Yang, J.; Andrade-Gordon, P.; Damiano, B. Characterization of functional urotensin II receptors in human skeletal muscle myoblasts: Comparison with angiotensin II receptors. Peptides 2005, 26, 683–690. [Google Scholar] [CrossRef]
- Vanhove, C.; Bankstahl, J.P.; Krämer, S.D.; Visser, E.; Belcari, N.; Vandenberghe, S. Accurate molecular imaging of small animals taking into account animal models, handling, anaesthesia, quality control and imaging system performance. EJNMMI Physics 2015, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Diallo, M.; Jarry, M.; Desrues, L.; Castel, H.; Chatenet, D.; Leprince, J.; Vaudry, H.; Tonon, M.C.; Gandolfo, P. [Orn5]URP acts as a pure antagonist of urotensinergic receptors in rat cortical astrocytes. Peptides 2008, 29, 813–819. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Nohata, N.; Goto, Y.; Gutkind, J.S. Onco-GPCR signaling and dysregulated expression of microRNAs in human cancer. J. Hum. Genet. 2017, 62, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Bernhardt, P.; Nilsson, O.; Ahlman, H.; Kölby, L.; Forssell-Aronsson, E. Differences in biodistribution between 99mTc-depreotide, 111In-DTPA-octreotide, and 177Lu-DOTA-Tyr3-octreotate in a small cell lung cancer animal model. Cancer Biother. Radiopharm. 2005, 20, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.Y.; Ilyosbek, S.; Lee, B.H.; Yi, K.Y.; Jung, Y.S. A novel urotensin II receptor antagonist, KR-36676, prevents ABCA1 repression via ERK/IL-1 beta pathway. Eur. J. Pharmacol. 2017, 803, 174–178. [Google Scholar] [CrossRef]
- Guidolin, D.; Albertin, G.; Oselladore, B.; Sorato, E.; Rebuffat, P.; Mascarin, A.; Ribatti, D. The pro-angiogenic activity of urotensin-II on human vascular endothelial cells involves ERK1/2 and PI3K signaling pathways. Regul. Pept. 2010, 162, 26–32. [Google Scholar] [CrossRef]
- Federico, A.; Zappavigna, S.; Dallio, M.; Misso, G.; Merlino, F.; Loguercio, C.; Novellino, E.; Grieco, P.; Caraglia, M. Urotensin-II receptor: A double identity receptor involved in vascoconstriction and in the development of digestive tract cancers and other tumors. Curr. Cancer Drug Targets 2017, 17, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Grieco, P.; Franco, R.; Bozzuto, G.; Toccacieli, L.; Sgambato, A.; Marra, M.; Zappavigna, S.; Migaldi, M.; Rossi, G.; Striano, S.; et al. Urotensin II receptor predicts the clinical outcome of prostate cancer patients and is involved in the regulation of motility of prostate adenocarcinoma cells. J. Cell. Biochem. 2011, 112, 341–353. [Google Scholar] [CrossRef]
- Langer, M.; La Bella, R.; Garcia-Garayoa, E.; Beck-Sickinger, A.G. 99mTc-labeled neuropeptide Y analogues as potential tumor imaging agents. Bioconjug. Chem. 2001, 12, 1028–1034. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Link, J.M.; Linden, H.M.; Sundararajan, L.; Krohn, K.A. Tumor receptor imaging. J. Nucl. Med. 2008, 49, 149S–163S. [Google Scholar] [CrossRef] [Green Version]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Mäcke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [Green Version]
- Radford, L.; Gallazzi, F.; Watkinson, L.; Carmack, T.; Berendzen, A.; Lewis, M.R.; Jurisson, S.S.; Papagiannopoulou, D.; Hennkens, H.M. Synthesis and evaluation of a 99mTc tricarbonyl-labeled somatostatin receptor-targeting antagonist peptide for imaging of neuroendocrine tumors. Nucl. Med. Biol. 2017, 47, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breeman, W.A.P.; de Jong, M.; Visser, T.J.; Erion, J.L.; Krenning, E.P. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Forrer, F.; Uusijärvi, H.; Waldherr, C.; Cremonesi, M.; Bernhardt, P.; Mueller-Brand, J.; Maecke, H.R. A comparison of 111In-DOTATOC and 111In-DOTATATE: Biodistribution and dosimetry in the same patients with metastatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1257–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldherr, C.; Pless, M.; Maecke, H.R.; Haldemann, A.; Mueller-Brand, J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: A clinical phase II study. Ann. Oncol. 2001, 12, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Cremonesi, M.; Grana, C.M.; Fazio, N.; Iodice, S.; Baio, S.M.; Bartolomei, M.; Lombardo, D.; Ferrari, M.E.; Sansovini, M.; et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: The IEO phase I-II study. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2125–2135. [Google Scholar] [CrossRef]
- Lacoeuille, F.; Arlicot, N.; Faivre-Chauvet, A. Targeted alpha and beta radiotherapy: An overview of radiopharmaceutical and clinical aspects. J. Nucl. Med. 2018, 42, 32–44. [Google Scholar] [CrossRef]
- Wulfert, S.; Kratochwil, C.; Choyke, P.L.; Afshar-Oromieh, A.; Mier, W.; Kauczor, H.U.; Schenk, J.P.; Haberkorn, U.; Giesel, F.L. Multimodal imaging for early functional response assessment of 90Y-177Lu-DOTATOC peptide receptor targeted radiotherapy with DW-MRI and 68Ga-DOTATOC-PET/CT. Mol. Imag. Biol. 2014, 16, 586–594. [Google Scholar] [CrossRef]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu- and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra- and extramedullary disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Lapa, C.; Herrmann, K.; Schirbel, A.; Hänscheid, H.; Lückerath, K.; Schottelius, M.; Kircher, M.; Werner, R.A.; Schreder, M.; Samnick, S.; et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed Multiple Myeloma. Theranostics 2017, 7, 1589–1597. [Google Scholar] [CrossRef] [Green Version]
- Bandari, R.P.; Jiang, Z.; Reynolds, T.S.; Bernskoetter, N.E.; Szczodroski, A.F.; Bassuner, K.J.; Kirkpatrick, D.L.; Rold, T.L.; Sieckman, G.L.; Hoffman, T.J.; et al. Synthesis and biological evaluation of copper-64 radiolabeled [DUPA-6-Ahx-(NODAGA)-5-Ava-BBN(7-14)NH2], a novel bivalent targeting vector having affinity for two distinct biomarkers (GRPr/PSMA) of prostate cancer. Nucl. Med. Biol. 2014, 41, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, T.J.S.; Schehr, R.; Liu, D.; Xu, J.; Miao, Y.; Hoffman, T.J.; Rold, T.L.; Lewis, M.R.; Smith, C.J. Characterization and evaluation of DOTA-conjugated Bombesin/RGD-antagonists for prostate cancer tumor imaging and therapy. Nucl. Med. Biol. 2015, 42, 99–108. [Google Scholar] [CrossRef] [PubMed]
- De León-Rodríguez, L.M.; Kovacs, Z. The Synthesis and Chelation Chemistry of DOTA-peptide Conjugates. Bioconjug. Chem. 2008, 19, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Roosenburg, S.; Laverman, P.; Joosten, L.; Cooper, M.S.; Kolenc-Peitl, P.K.; Foster, J.M.; Hudson, C.; Leyton, J.; Burnet, J.; Oyen, W.J.G.; et al. PET and SPECT imaging of a radiolabeled minigastrin analogue conjugated with DOTA, NOTA, and NODAGA and labeled with 64Cu, 68Ga, and 111In. Mol. Pharm. 2014, 11, 3930–3937. [Google Scholar] [CrossRef] [PubMed]
- Werle, M.; Bernkop-Schnürch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006, 30, 351–367. [Google Scholar] [CrossRef]
- Tatsi, A.; Maina, T.; Cescato, R.; Waser, B.; Krenning, E.P.; de Jong, M.; Cordopatis, P.; Reubi, J.C.; Nock, B.A. [DOTA]Somatostatin-14 analogs and their 111In-radioligands: Effects of decreasing ring-size on sst1–5 profile, stability and tumor targeting. Eur. J. Med. Chem. 2014, 73, 30–37. [Google Scholar] [CrossRef]
- Fani, M.; Braun, F.; Waser, B.; Beetschen, K.; Cescato, R.; Erchegyi, J.; Rivier, J.E.; Weber, W.A.; Maecke, H.R.; Reubi, J.C. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J. Nucl. Med. 2012, 53, 1481–1489. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shi, Z.; Yu, X.; Feng, P.; Wang, X.J. The effects of urotensin II on migration and invasion are mediated by NADPH oxidase-derived reactive oxygen species through the c-Jun N-terminal kinase pathway in human hepatoma cells. Peptides 2017, 88, 106–114. [Google Scholar] [CrossRef]
- Valkema, R.; Pauwels, S.A.; Kvols, L.K.; Kwekkeboom, D.J.; Jamar, F.; de Jong, M.; Barone, R.; Walrand, S.; Kooij, P.P.M.; Bakker, W.H.; et al. Long-Term Follow-Up of Renal Function After Peptide Receptor Radiation Therapy with 90Y-DOTA0,Tyr3-Octreotide and 177Lu-DOTA0, Tyr3-Octreotate. J. Nucl. Med. 2005, 46, 83–91. [Google Scholar]
- Imhof, A.; Brunner, P.; Marincek, N.; Briel, M.; Schindler, C.; Rasch, H.; Mäcke, H.R.; Rochlitz, C.; Müller-Brand, J.; Walter, M.A. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011, 29, 2416–2423. [Google Scholar] [CrossRef]
- Wei, L.; Petryk, J.; Gaudet, C.; Kamkar, M.; Gan, W.; Duan, Y.; Ruddy, T.D. Development of an inflammation imaging tracer, 111In-DOTA-DAPTA, targeting chemokine receptor CCR5 and preliminary evaluation in an ApoE−/− atherosclerosis mouse model. J. Nucl. Cardiol. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Thakur, M.L.; Marcus, C.S.; Saeed, S.; Pallela, V.; Minami, C.; Diggles, L.; Le Pham, H.; Ahdoot, R.; Kalinowski, E.A. 99mTc-labeled vasoactive intestinal peptide analog for rapid localization of tumors in humans. J. Nucl. Med. 2000, 41, 107–110. [Google Scholar] [PubMed]
- Bombardieri, E.; Ambrosini, V.; Aktolun, C.; Baum, R.P.; Bishof-Delaloye, A.; Del Vecchio, S.; Maffioli, L.; Mortelmans, L.; Oyen, W.; Pepe, G.; et al. 111In-pentetreotide scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Knigge, U.; Binderup, T.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Rasmussen, P.; Elema, D.; et al. 64Cu-DOTATATE PET for Neuroendocrine Tumors: A Prospective Head-to-Head Comparison with 111In-DTPA-Octreotide in 112 Patients. J. Nucl. Med. 2015, 56, 847–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valverde, I.E.; Vomstein, S.; Mindt, T.L. Toward the optimization of bombesin-based radiotracers for tumor targeting. J. Med. Chem. 2016, 59, 3867–3877. [Google Scholar] [CrossRef]
- Lim, J.C.; Cho, E.H.; Kim, J.J.; Choi, S.M.; Lee, S.Y.; Nam, S.S.; Park, U.J.; Park, S.H. Preclinical pharmacokinetic, biodistribution, imaging and therapeutic efficacy of 177Lu-Labeled glycated bombesin analogue for gastrin-releasing peptide receptor-positive prostate tumor targeting. Nucl. Med. Biol. 2015, 42, 234–241. [Google Scholar] [CrossRef]
- Malric, L.; Monferran, S.; Gilhodes, J.; Boyrie, S.; Dahan, P.; Skuli, N.; Sesen, J.; Filleron, T.; Kowalski-Chauvel, A.; Cohen-Jonathan Moyal, E.; et al. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: An update. Oncotarget 2017, 8, 86947–86968. [Google Scholar] [CrossRef] [Green Version]
- Tabatabai, G. The Role of Integrins in Angiogenesis. In Biochemical Basis and Therapeutic Implications of Angiogenesis; Springer: Cham, Switzerlands, 2017. [Google Scholar] [CrossRef]
- Taggart, M.D.; Rippaus, D.N.; Andreou, D.T.; Williams, M.J.; Wurdak, D.H.; Wronski, M.K.; Lorger, D.M. Site-specific phenotypes of cancer cells and macrophages in intracranial breast cancer metastases. Neuro Oncol. 2017, 19, 12–19. [Google Scholar] [CrossRef] [Green Version]







| Time in Plasma | Percent of Radiolabelled Peptide |
|---|---|
| 0 h | 98 ± 2 (%) |
| 1 h | 82 ± 2 (%) |
| 3 h | 71 ± 4 (%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poret, B.; Desrues, L.; Bonin, M.-A.; Pedard, M.; Dubois, M.; Leduc, R.; Modzelewski, R.; Decazes, P.; Morin, F.; Vera, P.; et al. Development of Novel 111-In-Labelled DOTA Urotensin II Analogues for Targeting the UT Receptor Overexpressed in Solid Tumours. Biomolecules 2020, 10, 471. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030471
Poret B, Desrues L, Bonin M-A, Pedard M, Dubois M, Leduc R, Modzelewski R, Decazes P, Morin F, Vera P, et al. Development of Novel 111-In-Labelled DOTA Urotensin II Analogues for Targeting the UT Receptor Overexpressed in Solid Tumours. Biomolecules. 2020; 10(3):471. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030471
Chicago/Turabian StylePoret, Benjamin, Laurence Desrues, Marc-André Bonin, Martin Pedard, Martine Dubois, Richard Leduc, Romain Modzelewski, Pierre Decazes, Fabrice Morin, Pierre Vera, and et al. 2020. "Development of Novel 111-In-Labelled DOTA Urotensin II Analogues for Targeting the UT Receptor Overexpressed in Solid Tumours" Biomolecules 10, no. 3: 471. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030471