Attenuation of Inflammatory Symptoms by Icariside B2 in Carrageenan and LPS-Induced Inflammation Models via Regulation of MAPK/NF-κB Signaling Cascades
Abstract
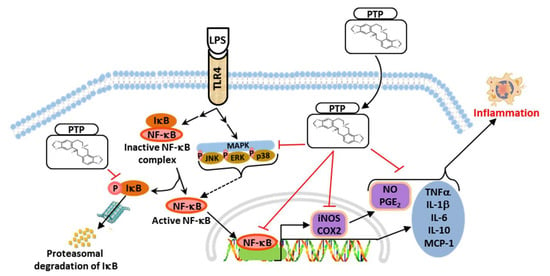
:1. Introduction
2. Materials and Methods
2.1. COX-2 Enzyme Inhibiton
2.2. Cell Viability and Nitric Oxide Determination
2.3. TNF-α, IL-1β, IL-6, and PGE2 Assays
2.4. CA-Induced Paw Edema
2.5. Transfection and Luciferase Assays for NF-kB
2.6. mRNA Analysis by a Semi-Quantitative Reverse Transcriptase-Polymerase Chain Reaction
2.7. Immunofluorescence Assay
2.8. Western Blotting Analysis
2.9. Molecular Docking Study
2.10. Statistical Analysis
3. Results
3.1. ICSB as a Major Ingredient in E. koreanum Nakai
3.2. Effect of ICSB on the Production of Inflammatory Mediators in LPS-Induced BV2 Cells
3.3. Effect of ICSB on Upstream Signaling for NF-κB Activation in LPS-Induced BV2 Cells
3.4. ICSB Attenuated MAPK Phosphorylation in LPS-Stimulated BV2 Cells
3.5. Inhibitory Effects of ICSB on CA-Induced Mouse Hind Paw Edema
3.6. Effect of ICSB on COX-2 Enzyme and Molecular Docking to the COX-2 Enzyme
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CNS | Central nervous system |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinase |
| ICR | Institute of Cancer Research |
| MAPK | Mitogen-activated protein kinase |
| PDB | Protein Data Bank |
References
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflammation 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Nam, Y.; Koo, J.Y.; Lim, D.; Park, J.; Ock, J.; Kim, J.; Suk, K.; Park, S.B. A small molecule binding HMGB1 and HMGB2 inhibits microglia-mediated neuroinflammation. Nat. Chem. Biol. 2014, 10, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Medicine. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Sautebin, L.; De Sarro, G.; Costantino, G.; Rombola, L.; Mazzon, E.; Ialenti, A.; De Sarro, A.; Ciliberto, G.; Di Rosa, M.; et al. Role of IL-6 in the pleurisy and lung injury caused by carrageenan. J. Immunol. 1999, 163, 5094–5104. [Google Scholar]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Reviews. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Khan, S.; Shehzad, O.; Jin, H.G.; Woo, E.R.; Kang, S.S.; Baek, S.W.; Kim, J.; Kim, Y.S. Anti-inflammatory mechanism of 15,16-epoxy-3alpha-hydroxylabda-8,13(16),14-trien-7-one via inhibition of LPS-induced multicellular signaling pathways. J. Nat. Prod. 2012, 75, 67–71. [Google Scholar] [CrossRef]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.; Weng, Y.; Yu, Y.; Zhang, D.; Fan, W.; Dai, R.; Hu, Z. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci. 2004, 74, 2467–2478. [Google Scholar] [CrossRef]
- Meng, F.-H.; Li, Y.-B.; Xiong, Z.-L.; Jiang, Z.-M.; Li, F.-M. Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine: Int. J. Phytother. Phytopharm. 2005, 12, 189–193. [Google Scholar] [CrossRef]
- Jiang, J.; Song, J.; Jia, X.-b. Phytochemistry and ethnopharmacology of Epimedium L. species. Chin. Herb. Med. 2015, 7, 204–222. [Google Scholar] [CrossRef]
- Chun, K.; Alam, M.B.; Son, H.-U.; Lee, S.-H. Effect of Novel Compound LX519290, a Derivative of l-allo Threonine, on Antioxidant Potential in Vitro and in Vivo. Int. J. Mol. Sci. 2016, 17, 1451. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.B.; Bajpai, V.K.; Lee, J.; Zhao, P.; Byeon, J.-H.; Ra, J.-S.; Majumder, R.; Lee, J.S.; Yoon, J.-I.; Rather, I.A. Inhibition of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP-Kinase mediated MITF downregulation and the proteasomal degradation of tyrosinase. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Alam, M.B.; Ju, M.K.; Kwon, Y.G.; Lee, S.H. Protopine attenuates inflammation stimulated by carrageenan and LPS via the MAPK/NF-kappaB pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 131, 110583. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.L.; Lin, L.L.; Zhang, L.; Li, L. Epimedium flavonoids ameliorate experimental autoimmune encephalomyelitis in rats by modulating neuroinflammatory and neurotrophic responses. Neuropharmacology 2012, 63, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Kiem, P.V.; Cuong, L.C.; Tai, B.H.; Nhiem, N.X.; Anh, H.L.; Quang, T.H.; Ngan, N.T.; Oh, H.; Kim, Y.C. New Lignans from Antidesma hainanensis Inhibit NO Production in BV2 Microglial Cells. Chem. Pharm. Bull. 2016, 64, 1707–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, H.M.; Yu, J.S.; Khan, Z.; Subedi, L.; Ko, Y.J.; Lee, I.K.; Park, W.S.; Chung, S.J.; Ahn, M.J.; Kim, S.Y.; et al. Chemical constituents of the root bark of Ulmus davidiana var. japonica and their potential biological activities. Bioorganic Chem. 2019, 91, 103145. [Google Scholar] [CrossRef]
- Lee, M.K.; Jeon, H.Y.; Lee, K.Y.; Kim, S.H.; Ma, C.J.; Sung, S.H.; Lee, H.S.; Park, M.J.; Kim, Y.C. Inhibitory constituents of Euscaphis japonica on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Planta Med. 2007, 73, 782–786. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet. Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Li, C.; Yang, D.; Cao, X.; Wang, F.; Jiang, H.; Guo, H.; Du, L.; Guo, Q.; Yin, X. LFG-500, a newly synthesized flavonoid, attenuates lipopolysaccharide-induced acute lung injury and inflammation in mice. Biochem. Pharm. 2016, 113, 57–69. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, H.J.; Lee da, Y.; Kim, J.S.; Kim, D.H.; Lee, H.J.; Kim, H.D.; Jeon, R.; Ryu, J.H. In vitro anti-inflammatory activity of lignans isolated from Magnolia fargesii. Bioorganic Med. Chem. Lett. 2009, 19, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, L.; Liu, K. In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. J. Ethnopharmacol. 2009, 122, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, T.Y.; Hwang, T.L. Neolignans, a coumarinolignan, lignan derivatives, and a chromene: Anti-inflammatory constituents from Zanthoxylum avicennae. J. Nat. Prod. 2008, 71, 212–217. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, P.; Gadhwal, M.K.; Joshi, U.; Shetgiri, P. Modeling of COX-2 inhibitory activity of flavonoids. Int. J. Pharm. Pharm. Sci. 2011, 3, 33–40. [Google Scholar]
- Wang, J.P.; Zhou, Y.M.; Ye, Y.J.; Shang, X.M.; Cai, Y.L.; Xiong, C.M.; Wu, Y.X.; Xu, H.X. Topical anti-inflammatory and analgesic activity of kirenol isolated from Siegesbeckia orientalis. J. Ethnopharmacol. 2011, 137, 1089–1094. [Google Scholar] [CrossRef]
- Corsi, L.; Zavatti, M.; Geminiani, E.; Zanoli, P.; Baraldi, M. Anti-inflammatory activity of the non-peptidyl low molecular weight radical scavenger IAC in carrageenan-induced oedema in rats. J. Pharm. Pharmacol. 2011, 63, 417–422. [Google Scholar] [CrossRef]
- Lee, C.W.; Park, S.M.; Zhao, R.; Lee, C.; Chun, W.; Son, Y.; Kim, S.H.; Jung, J.Y.; Jegal, K.H.; Cho, I.J.; et al. Hederagenin, a major component of Clematis mandshurica Ruprecht root, attenuates inflammatory responses in RAW 264.7 cells and in mice. Int. Immunopharmacol. 2015, 29, 528–537. [Google Scholar] [CrossRef]
- Dhawan, P.; Richmond, A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J. Biol. Chem. 2002, 277, 7920–7928. [Google Scholar] [CrossRef] [Green Version]
- Ci, X.; Ren, R.; Xu, K.; Li, H.; Yu, Q.; Song, Y.; Wang, D.; Li, R.; Deng, X. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-kappaB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation 2010, 33, 126–136. [Google Scholar] [CrossRef]
- Lu, Y.; Suh, S.J.; Kwak, C.H.; Kwon, K.M.; Seo, C.S.; Li, Y.; Jin, Y.; Li, X.; Hwang, S.L.; Kwon, O.; et al. Saucerneol F, a new lignan, inhibits iNOS expression via MAPKs, NF-kappaB and AP-1 inactivation in LPS-induced RAW264.7 cells. Int. Immunopharmacol. 2012, 12, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part II. modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handy, R.L.; Moore, P.K. A comparison of the effects of L-NAME, 7-NI and L-NIL on carrageenan-induced hindpaw oedema and NOS activity. Br. J. Pharmacol. 1998, 123, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Kim, S.C.; Shin, B.Y.; Jin, S.H.; Jo, M.J.; Jegal, K.H.; Kim, Y.W.; Lee, J.R.; Ku, S.K.; Cho, I.J.; et al. O-Methylated flavonol isorhamnetin prevents acute inflammation through blocking of NF-kappaB activation. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 59, 362–372. [Google Scholar] [CrossRef]
- Borthakur, A.; Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am. J. Physiology. Gastrointest. Liver Physiol. 2007, 292, G829–G838. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.B.; Kwon, Y.-G.; Simu, S.Y.; Abrar Shahriyar, S.; Lee, S.H. Attenuation of Inflammatory Symptoms by Icariside B2 in Carrageenan and LPS-Induced Inflammation Models via Regulation of MAPK/NF-κB Signaling Cascades. Biomolecules 2020, 10, 1037. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10071037
Alam MB, Kwon Y-G, Simu SY, Abrar Shahriyar S, Lee SH. Attenuation of Inflammatory Symptoms by Icariside B2 in Carrageenan and LPS-Induced Inflammation Models via Regulation of MAPK/NF-κB Signaling Cascades. Biomolecules. 2020; 10(7):1037. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10071037
Chicago/Turabian StyleAlam, Md Badrul, Yoon-Gyung Kwon, Shakina Yesmin Simu, Sk Abrar Shahriyar, and Sang Han Lee. 2020. "Attenuation of Inflammatory Symptoms by Icariside B2 in Carrageenan and LPS-Induced Inflammation Models via Regulation of MAPK/NF-κB Signaling Cascades" Biomolecules 10, no. 7: 1037. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10071037