Understanding the Biosynthetic Changes that Give Origin to the Distinctive Flavor of Sotol: Microbial Identification and Analysis of the Volatile Metabolites Profiles During Sotol (Dasylirion sp.) Must Fermentation
Abstract
:1. Introduction
2. Materials and Methods
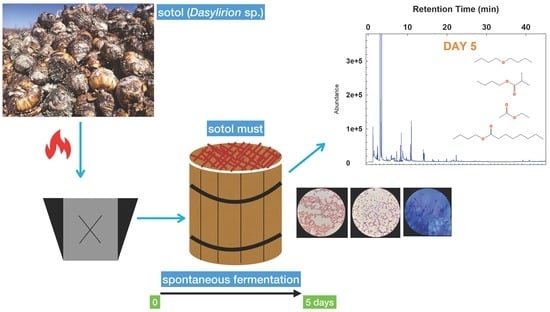
2.1. Fermented Sotol Must Samples
2.2. Enumeration of Microorganisms
2.3. Microbial Consortium Identification and Data Analysis
2.4. Total Reducing Sugars (TRS) Determination
2.5. Volatile Metabolites Analysis by Headspace Solid-Phase Microextraction (HS-SPME)
2.6. Alcoholic Fermentation Efficiency
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Kinetics
3.2. Microbial Identification
3.3. Variation of Volatile Metabolites Profiles During Sotol Must Fermentation
3.4. PCA Analysis of Aroma Compounds Variation During Sotol Must Fermentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IMPI. Denominaciones de Origen: Orgullo de México; Ed Pax México: Mexico City, Mexico, 2016; Volume 1. [Google Scholar]
- Tristán-Sierra, J.S.; Lara-Macías, C.R.; Carillo-Romo, R.; Mendoza-Castillo, A.; Morales-Nieto, C.; Royo-Marquez, M.H. Los Sotoles (Dasylirion sp.) de Chihuahua. Natl. Inst. For. Agric. Livest. Res. 2008, 2008, 58. [Google Scholar]
- De La Garza-Toledo, H.; Martinez, M.; Lara, L.; Rodriguez-Herrera, R.; Rodriguez-Martinez, J.; Aguilar, C.N. Production of a Mexican Alcoholic Beverage: Sotol. Res. J. Biol. Sci. 2008, 3, 566–571. [Google Scholar]
- SCFI. Alcoholic Beverages-Sotol-Specifications and Test Methods; NOM-159-SCFI-2004; Commerce and Industrial Development: Mexico City, Mexico, 2004.
- De la Garza, H.; Buenrostro, J.; Reyes-Vega, M.; Rodriguez, R.; Martinez, D.G.; Aguilar, C.N. Chemical Profile of Sotol Analyzed by Solid Phase Microextraction-Gas Chromatography. Am. J. Agric. Biol. Sci. 2010, 5, 261–268. [Google Scholar] [CrossRef]
- Hoffman, C.S.; Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 1987, 57, 267–272. [Google Scholar] [CrossRef]
- La Duc, M.T.; Vaishampayan, P.; Nilsson, H.R.; Torok, T.; Venkateswaran, K. Pyrosequencing-Derived Bacterial, Archaeal, and Fungal Diversity of Spacecraft Hardware Destined for Mars. Appl. Environ. Microbiol. 2012, 78, 5912. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.; Fierer, N.; Peña, A.; Goodrich, J.; Gordon, J.; et al. QIIME allows analysis of high–throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Edgar, R.; Haas, B.; Clemente, J.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Altschul, S.; Gish, W.; Miller, W.; Myers, E.; Lipman, D. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Bernfeld, P. Enzymes of starch degradation and synthesis. Adv. Enzymol. Relat. Subj. Biochem. 1951, 12, 379–428. [Google Scholar] [CrossRef]
- Lappe-Oliveras, P.; Moreno-Terrazas, R.; Arrizon-Gavino, J.; Herrera-Suarez, T.; Garcia-Mendoza, A.; Gschaedler-Mathis, A. Yeasts associated with the production of Mexican alcoholic nondistilled and distilled Agave beverages. FEMS Yeast Res. 2008, 8, 1037–1052. [Google Scholar] [CrossRef]
- Lachance, M.A. Yeast communities in a natural tequila fermentation. Antonie Van Leeuwenhoek 1995, 68, 151–160. [Google Scholar] [CrossRef]
- Verdugo Valdez, A.; Segura Garcia, L.; Kirchmayr, M.; Ramírez Rodríguez, P.; González Esquinca, A.; Coria, R.; Gschaedler Mathis, A. Yeast communities associated with artisanal mezcal fermentations from Agave salmiana. Antonie Van Leeuwenhoek 2011, 100, 497–506. [Google Scholar] [CrossRef]
- Ramos, C.L.; de Almeida, E.G.; Pereira, G.V.D.M.; Cardoso, P.G.; Dias, E.S.; Schwan, R.F. Determination of dynamic characteristics of microbiota in a fermented beverage produced by Brazilian Amerindians using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2010, 140, 225–231. [Google Scholar] [CrossRef] [PubMed]
- John, W.A.; Böttcher, N.L.; Aßkamp, M.; Bergounhou, A.; Kumari, N.; Ho, P.-W.; D’Souza, R.N.; Nevoigt, E.; Ullrich, M.S. Forcing fermentation: Profiling proteins, peptides and polyphenols in lab-scale cocoa bean fermentation. Food Chem. 2019, 278, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Sannino, C.; Francesca, N.; Corona, O.; Settanni, L.; Cruciata, M.; Moschetti, G. Effect of the natural winemaking process applied at industrial level on the microbiological and chemical characteristics of wine. J. Biosci. Bioeng. 2013, 116, 347–356. [Google Scholar] [CrossRef] [PubMed]
- De Roos, J.; De Vuyst, L. Microbial acidification, alcoholization, and aroma production during spontaneous lambic beer production. J. Sci. Food Agric. 2019, 99, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronek, M.; Fiedurek, J. Selection of biochemical mutants of Aspergillus niger resistant to some abiotic stresses with increased inulinase production. J. Appl. Microbiol. 2003, 95, 686–692. [Google Scholar] [CrossRef]
- Sánchez-Madrigal, M.Á.; Viesca-Nevárez, S.L.; Quintero-Ramos, A.; Amaya-Guerra, C.A.; Meléndez-Pizarro, C.O.; Contreras-Esquivel, J.C.; Talamás-Abbud, R. Optimization of the enzyme-assisted extraction of fructans from the wild sotol plant (Dasylirion wheeleri). Food Biosci. 2018, 22, 59–68. [Google Scholar] [CrossRef]
- Rawat, H.K.; Ganaie, M.A.; Kango, N. Production of inulinase, fructosyltransferase and sucrase from fungi on low-value inulin-rich substrates and their use in generation of fructose and fructo-oligosaccharides. Antonie Van Leeuwenhoek 2015, 107, 799–811. [Google Scholar] [CrossRef]
- Kirchmayr, M.R.; Segura-García, L.E.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; de la Rosa, M.; Gschaedler Mathis, A. Impact of environmental conditions and process modifications on microbial diversity, fermentation efficiency and chemical profile during the fermentation of Mezcal in Oaxaca. LWT Food Sci. Techno. 2017, 79, 160–169. [Google Scholar] [CrossRef]
- González-Robles, I.W.; Estarrón-Espinosa, M.; Díaz-Montaño, D.M. Fermentative capabilities and volatile compounds produced by Kloeckera/Hanseniaspora and Saccharomyces yeast strains in pure and mixed cultures during Agave tequilana juice fermentation. Antonie van Leeuwenhoek 2015, 108, 525–536. [Google Scholar] [CrossRef]
- Bayrock, D.P.; Ingledew, W.M. Inhibition of yeast by lactic acid bacteria in continuous culture: Nutrient depletion and/or acid toxicity? J. Ind. Microbiol. Biotechnol. 2004, 31, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Aldrete-Tapia, J.A.; Escalante-Minakata, P.; Martínez-Peniche, R.A.; Tamplin, M.L.; Hernández-Iturriaga, M. Yeast and bacterial diversity, dynamics and fermentative kinetics during small-scale tequila spontaneous fermentation. Food Microbiol. 2020, 86, 103339. [Google Scholar] [CrossRef]
- Díaz-Montaño, D.M.; Délia, M.-L.; Estarrón-Espinosa, M.; Strehaiano, P. Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enzyme Microb. Technol. 2008, 42, 608–616. [Google Scholar] [CrossRef]
- Díaz-Montaño, D.; Favela-Torres, E.; Cordova, J. Improvement of growth, fermentative efficiency and ethanol tolerance of Kloeckera africana during the fermentation of Agave tequilana juice by addition of yeast extract. J. Sci. Food Agric. 2010, 90, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, I. Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards Nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Walker, G.M.; Hill, A.E. Saccharomyces cerevisiae in the Production of Whisky. Beverages 2016, 2, 38. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Rossi, C.; Pepe, A.; Compagnone, E.; Paparella, A. Interaction between Galactomyces geotrichum KL20B, Lactobacillus plantarum LAT3 and Enterococcus faecalis KE06 during Milk Fermentation. Fermentation 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of aromatic compounds precursors during fermentation of Criollo and Forastero cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Junqueira, A.C.; de Melo Pereira, G.V.; Coral Medina, J.D.; Alvear, M.C.R.; Rosero, R.; de Carvalho Neto, D.P.; Enríquez, H.G.; Soccol, C.R. First description of bacterial and fungal communities in Colombian coffee beans fermentation analysed using Illumina-based amplicon sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phaff, H.J.; Blue, J.; Hagler, A.N.; Kurtzman, C.P. Dipodascus starmeri sp. nov., a New Species of Yeast Occurring in Cactus Necroses. Int. J. Syst. Evol. Microbiol. 1997, 47, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Alvarez, J.; Neto, D.P.D.C.; Thomaz-Soccol, V.; Tanobe, V.; Rogez, H.; Góes-Neto, A.; Soccol, C. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT Food Sci. Technol. 2017, 84, 290–297. [Google Scholar] [CrossRef]
- Gaglio, R.; Alfonzo, A.; Francesca, N.; Corona, O.; Di Gerlando, R.; Columba, P.; Moschetti, G. Production of the Sicilian distillate “Spiritu re fascitrari” from honey by-products: An interesting source of yeast diversity. Int. J. Food Microbiol. 2017, 261, 62–72. [Google Scholar] [CrossRef]
- Yan, S.; Tong, Q.; Guang, J. Yeast dynamics and changes in volatile compounds during the fermentation of the traditional Chinese strong-flavor Daqu. LWT 2019, 106, 57–63. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Chen, L. Diversity of yeast species during fermentative process contributing to Chinese Maotai-flavor liquor making. Lett. Appl. Microbiol. 2012, 55, 301–307. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y. Microbial succession and metabolite changes during the fermentation of Chinese light aroma-style liquor. J. Inst. Brew. 2019, 125, 162–170. [Google Scholar] [CrossRef]
- Granchi, L.; Ganucci, D.; Buscioni, G.; Mangani, S.; Guerrini, S. The Biodiversity of Saccharomyces cerevisiae in Spontaneous Wine Fermentation: The Occurrence and Persistence of Winery-Strains. Fermentation 2019, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Yamane, Y.-I.; Fujita, J.; Izuwa, S.; Fukuchi, K.; Shimizu, R.-I.; Hiyoshi, A.; Fukuda, H.; Mikami, S.; Kizaki, Y.; Wakabayashi, S. Properties of cellulose-degrading enzymes from Aspergillus oryzae and their contribution to material utilization and alcohol yield in sake mash fermentation. J. Biosci. Bioeng. 2002, 93, 479–484. [Google Scholar] [CrossRef]
- De Oliveira Rodrigues, P.; Gurgel, L.V.A.; Pasquini, D.; Badotti, F.; Góes-Neto, A.; Baffi, M.A. Lignocellulose-degrading enzymes production by solid-state fermentation through fungal consortium among Ascomycetes and Basidiomycetes. Renew. Energy 2020, 145, 2683–2693. [Google Scholar] [CrossRef]
- De Araújo, J.A.; Ferreira, N.R.; da Silva, S.H.M.; Oliveira, G.; Monteiro, R.C.; Alves, Y.F.M.; Lopes, A.S. Filamentous fungi diversity in the natural fermentation of Amazonian cocoa beans and the microbial enzyme activities. Ann. Microbiol. 2019, 69, 975–987. [Google Scholar] [CrossRef]
- Das, D.; Selvaraj, R.; Ramananda Bhat, M. Optimization of inulinase production by a newly isolated strain Aspergillus flavus var. flavus by solid state fermentation of Saccharum arundinaceum. Biocatal. Agric. Biotechnol. 2019, 22, 101363. [Google Scholar] [CrossRef]
- Banjo, T.; Kareem, S.; Akinduti, P.; Popoola, T.; Akinloye, O. Optimization and production of ascorbic acid by fusant cell of Aspergillus flavus and Aspergillus tamarii. J. King Saud Univ. Sci. 2019, 31, 931–936. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Biotechnological production of flavors and fragrances. Appl. Microbiol. Biotechnol. 1998, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kays, S. Contribution of Volatile Compounds to the Characteristic Aroma of Baked ’Jewel’ Sweetpotatoes. J. Am. Soc. Hortic. Sci. 2000, 125, 638–643. [Google Scholar] [CrossRef]
- Samaga, P.V.; Rai, V.R.; Rai, K.M.L. Bionectria ochroleuca NOTL33—An endophytic fungus from Nothapodytes foetida producing antimicrobial and free radical scavenging metabolites. Ann. Microbiol. 2014, 64, 275–285. [Google Scholar] [CrossRef]
- Wang, B.; You, J.; King, J.B.; Cai, S.; Park, E.; Powell, D.R.; Cichewicz, R.H. Polyketide Glycosides from Bionectria ochroleuca Inhibit Candida albicans Biofilm Formation. J. Nat. Prod. 2014, 77, 2273–2279. [Google Scholar] [CrossRef]
- Siwarungson, N.; Ohsugi, M. Purification and Properties of a Fibrinolytic Enzyme of Ascomycetes, Bionectria ochroleuca. Purif. Prop. Fibrinolytic Enzyme Ascomycetes Bionectria Ochroleuca 2007, 32, 73–80. [Google Scholar]
- Christoph, N.; Bauer-Christoph, C. Flavors and Fragrances: Chemistry, Bioprocessing and Sustainability. In Flavors and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 648. [Google Scholar]
- Lea, A.G.H.; Piggott, J.R. Fermented Beverage Production, 2nd ed.; Kluwer Academic: New York, NY, USA, 2003; pp. 358–360. ISBN 0-306-47275-9. [Google Scholar]
- Bloem, A.; Lonvaud-Funel, A.; de Revel, G. Hydrolysis of glycosidically bound flavor compounds from oak wood by Oenococcus oeni. Food Microbiol. 2008, 25, 99–104. [Google Scholar] [CrossRef]
- Wanikawa, A.; Hosoi, K.; Shoji, H.; Nakagawa, K.I. Estimation of the distribution of enantiomers of γ-decalactone and γ-dodecalactone in malt whisky. J. Inst. Brewing. 2001, 107, 253–259. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Zhang, Q.; Zhong, C.-H.; Guo, M.-Q. Volatile fingerprints and biomarkers of three representative kiwifruit cultivars obtained by headspace solid-phase microextraction gas chromatography mass spectrometry and chemometrics. Food Chem. 2019, 271, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Rassem, H.H.A.; Nour, A.H.; Rosli, M.Y. GC-MS analysis of bioactive constituents of Hibiscus flower. Aust. J. Basic Appl. Sci. 2017, 11, 91–97. [Google Scholar]
- Xu, M.; Jin, Z.; Lan, Y.; Rao, J.; Chen, B. HS-SPME-GC-MS/olfactometry combined with chemometrics to assess the impact of germination on flavor attributes of chickpea, lentil, and yellow pea flours. Food Chem. 2019, 280, 83–95. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Ragazzo-Sanchez, J.A.; Chalier, P.; Chevalier-Lucia, D.; Calderon-Santoyo, M.; Ghommidh, C. Off-flavors detection in alcoholic beverages by electronic nose coupled to GC. Sens. Actuators B Chem. 2009, 140, 29–34. [Google Scholar] [CrossRef]
- Perfumers-World. Product Search. Available online: https://perfumersworld.com/index.php (accessed on 20 November 2019).
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between Varietal Amino Acid Profile of Grapes and Wine Aromatic Composition. Experiments with Model Solutions and Chemometric Study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef]
- Romano, P.; Ciani, M.; Fleet, G.H. Yeasts in the Production of Wine; Springer: New York, NY, USA, 2019; p. 515. [Google Scholar]
- Furdíková, K.; Makyšová, K.; Spánik, I. Effect of Indigenous S. cerevisiae Strains on Higher Alcohols, Volatile Acids, and Esters in Wine. Czech J. Food Sci. 2017, 35, 2017–2131. [Google Scholar] [CrossRef]
- Fang, C.; Du, H.; Jia, W.; Xu, Y. Compositional Differences and Similarities between Typical Chinese Baijiu and Western Liquor as Revealed by Mass Spectrometry-Based Metabolomics. Metabolites 2019, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Grumezescu, A.; Holban, A.M. Fermented Beverages; Woodhead Publishing: Duxford, UK, 2019; Volume 5, pp. 226–233. ISBN 978-0-12-815703-9. [Google Scholar]
- Burdock, G.A. Encyclopedia of Food & Color Additives; CRC Press: Boca Raton, FL, USA, 1997; Volume 2, pp. 1454–1455. ISBN 978-0-8493-9416-4. [Google Scholar]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]




| Compound | RT (Min) | Flavor Descriptor a | Days of Fermentation | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||
| Alcohols | ||||||||
| 2-methylpropan-1-ol | 6.86 | Ethereal, winey | - | 28.11 ± 2.38 | - | - | 33.45 ± 2.41 | - |
| 2-ethylcyclobutan-1-ol | 9.64 | - | - | - | 18.82 ± 0.06 a | 18.43 ± 0.35 a | - | |
| 3-methylbutan-1-ol | 10.81 | Fruity, pungent | - | 39.62 ± 5.14 b | 61.31 ± 3.18 a | 57.63 ± 1.22 a | - | - |
| 3-bromopentan-2-ol | 16.38 | - | - | - | 9.48 ± 0.18 | - | - | |
| 6-amino-2-methylheptan-2-ol | 20.91 | - | 8.81 ± 0.23 | - | - | - | - | |
| octan-1-ol | 22.33 | Green leaves | 35.28 ± 0.53 b | 44.76 ± 1.53 a | 38.60 ± 3.73 b | 36.80 ± 1.26 b | 9.56 ± 0.18 d | 29.59 ± 0.26 c |
| (2R)-6-methyl-2-[(1R)-4-methylcyclohex-3-en-1-yl]hept-5-en-2-ol | 23.62 | Diesel | - | 10.46 ± 0.10 c | 12.94 ± 1.58 b | 18.18 ± 0.76 a | 18.06 ± 0.57 a | - |
| (+)-Alpha-bisabolol | 41.02 | - | - | 9.35 ± 0.24 | - | - | - | |
| (2R,3R,4R,5S)-hexane-1,2,3,4,5,6-hexol | 45.85 | Sweet | - | - | 10.75 ± 0.38 | - | - | - |
| Aldehydes | ||||||||
| butanal | 2.39 | Cheese, fruity | 66.92 ± 7.37 a | 61.78 ± 12.21 a | 29.23 ± 1.44 b | 18.51 ± 2.21b | - | 31.69 ± 1.20 b |
| 2-ethyl hexanal | 4.82 | 24.35 ± 1.53 | - | - | - | - | - | |
| 3-hexylimino-2-nitropropanal | 10.12 | - | - | - | - | - | 10.39 ± 0.20 | |
| 1,6,6-trimethylbicyclo [2.1.1]hexane-5-carbaldehyde | 26.82 | 10.88 ± 0.57 a | - | - | - | 10.47 ± 1.11 a | ||
| 3-[(E)-hydroxyiminomethyl]phenol | 40.82 | - | - | - | 36.67 ± 2.78 a | 13.99 ± 0.89 b | 11.03 ± 0.63 b | |
| 3,6-dimethyl-3,3a,4,5-tetrahydro-2H-indene-1-carbaldehyde | 45.86 | - | - | - | 10.51 ± 0.41 | - | - | |
| Acids | ||||||||
| 2-(carbamoylamino)-2-oxoacetic acid | 4.30 | 11.71 ± 0.20 | - | - | - | - | - | |
| acetohydrazide | 4.33 | - | - | - | - | 8.71 ± 0.16 | - | |
| 2-hydroxyacetic acid | 5.12 | Vinegar | - | - | 10.89 ± 0.34 a | 10.33 ± 0.39 a | 10.51 ± 0.13 a | - |
| (2R,3R)-2-amino-3-hydroxybutanoic acid | 8.02 | - | - | - | 10.68 ± 0.31 a | 10.74 ± 0.44 a | - | |
| 1,3,4-trihydroxy-5-oxocyclohexane-1-carboxylic acid | 21.17 | - | - | - | 16.06 ± 12.01 | - | - | |
| 2-(propan-2-ylcarbamoylamino)acetic acid | 27.19 | - | 11.06 ± 0.55 | - | - | - | - | |
| 2-(carbamoylamino)-2-oxoacetic acid | 40.81 | - | - | - | - | - | 13.75 ± 0.12 | |
| Ethers | ||||||||
| 1-butoxybutane | 3.43 | Diesel | 473.13 ± 29.80 a | 432.21 ± 17.16 a,b | 24.96 ± 1.58 d | 386.95 ± 26.06 b,c | 334.51 ± 19.23 c | 476.67 ± 22.96 a |
| 1-[(E)-but-1-enoxy]butane | 4.66 | Sulphur | 41.20 ± 1.18 b | 49.94 ± 3.94 a | 12.05 ± 1.25 d | 20.79 ± 1.90 c | 19.17 ± 3.25 c | 17.52 ± 0.99 c,d |
| 5-ethyl-2,4-dipropyl-1,3-dioxane | 17.83 | - | 15.28 ± 1.04 ab | 17.64 ± 1.11 a | 10.34 ± 0.52 c | 14.51 ± 1.54 b | - | 12.73 ± 1.07 bc |
| Esters | ||||||||
| ethenyl formate | 1.52 | Floral | - | 156.64 ± 27.28 a | 101.65 ± 12.71 b | 91.84 ± 7.31 b | 120.89 ± 6.83 a | 79.94 ± 13.68 b |
| ethyl acetate | 2.48 | Fruity, pineapple | - | - | 19.86 ± 1.38 b | 17.92 ± 0.20 c | 16.48 ± 0.19 d | 25.63 ± 0.13 a |
| ethyl ethanimidate | 5.09 | Floral | - | - | - | - | - | 20.97 ± 0.35 |
| butan-2-yl nitrite | 7.45 | - | - | - | 10.46 ± 0.24 b | 10.02 ± 0.34 b | 11.27 ± 0.33 a | |
| butyl 2-methylpropanoate | 8.18 | Fruity | 94.99 ± 2.58 a | 63.36 ± 5.21 b | 17.75 ± 0.57 d | 59.30 ± 1.22 b,c | 50.85 ± 7.07 c | 50.51 ± 4.64 c |
| pentyl prop-2-enoate | 10.79 | 22.13 ± 1.74 | - | - | - | - | - | |
| butyl butanoate | 10.96 | Cheese | 107.56 ± 5.61 b | 142.67 ± 6.72 a | 40.13 ± 3.15 c | 109.97 ± 12.82 b | - | 106.3 ± 4.60 b |
| ethyl 2-hydroxypropanoate | 16.36 | - | - | - | - | 12.64 ± 0.24 b | 17.14 ± 1.30 a | |
| bis(trimethylsilyl) oxalate | 18.37 | Green | 17.99 ± 0.58 | - | - | - | - | - |
| ethyl octanoate | 19.88 | Fruity, winey | - | 24.20 ± 2.56 a | 14.49 ± 1.07 b | - | - | 17.55 ± 0.39 c |
| octyl 2-methylpropanoate | 23.39 | Vinegar | - | 14.65 ± 0.62 | - | - | - | - |
| octyl ester | 23.41 | Waxy | 14.73 ± 1.41 | - | - | - | - | - |
| thanedioic acid, bis(trimethylsilyl) ester | 25.45 | 75.12 ± 12.50 | - | - | - | - | - | |
| butyl octanoate | 26.99 | Creamy | - | 13.35 ± 1.21 | - | - | - | - |
| Hydrocarbons | ||||||||
| 4-methyl-2,3,4,5,6,7-hexahydro-1H-indene | 22.91 | Fruity | - | 10.49 ± 0.42 a | 10.53 ± 0.56 a | 10.19 ± 0.26 a | - | 10.05 ± 0.35 a |
| 3,5-dimethyl-1,2,4-trioxolane | 26.40 | - | - | - | - | - | 9.83 ± 0.15 | |
| 4,4-dimethyl-8-methylidene-1-oxaspiro[2.5]octane | 26.82 | - | - | - | 10.65 ± 0.38 | - | - | |
| Amines | ||||||||
| 2-(aziridin-1-yl)ethanamine | 1.26 | Fishy | 126.19 ± 26.0 a | 94.99 ± 3.62 a | 102.99 ± 10.89 a | 50.21 ± 2.05 b | - | - |
| octan-2-amine | 1.53 | 65.53 ± 10.29 | - | - | - | - | - | |
| dimethyl(trimethylsilyloxy)silicon | 2.22 | - | - | 12.44 ± 0.55 b | 19.95 ± 0.42 a | 19.54 ± 0.50 a | 19.75 ± 0.99 a | |
| N-methyl-N-(2-methylpropyl)nitrous amide | 5.94 | - | - | - | - | - | 22.01 ± 2.0 | |
| (2R,3R)-2-amino-3-hydroxybutanoic acid | 7.99 | - | 18.84 ± 1.17 | - | - | - | - | |
| 3,4-diamino-4-oxobutanoic acid | 19.90 | 11.32 ± 0.46 b | - | - | 10.45 ± 0.18 c | 16.17 ± 0.74 a | - | |
| Alkenes | ||||||||
| 1-[(E)-but-2-enoxy]butane | 5.84 | Diesel | 43.84 ± 1.72 b | 52.40 ± 3.90 a | - | 23.17 ± 1.68 c | 25.95 ± 1.11 c | 27.41 ± 0.54 c |
| 1,4-dimethoxybenzene | 39.20 | Sweet, green | - | - | - | 9.82 ± 0.21 b | 9.56 ± 0.35 b | 10.78 ± 0.25 a |
| Acetals | ||||||||
| 4-methyl-2-pentyl-1,3-dioxolane | 7.23 | Fruity, sweet | 41.18 ± 3.51 a | 40.76 ± 4.18 a | 38.17 ± 4.74 a | 35.66 ± 2.94 a | 33.45 ± 2.41 a | 33.32 ± 1.79 a |
| 2-butyl-4-methyl-1,3-dioxolane | 7.89 | Fatty | 15.98 ± 1.02 b,c | 46.98 ± 1.55 a | 17.09 ± 1.67 b | 15.26 ± 0.47 bc | 13.45 ± 1.27 c | 14.40 ± 0.63 bc |
| Other compounds | ||||||||
| 3,3-dimethyldiaziridine | 4.82 | - | 27.57 ± 1.93 a | - | 11.31 ± 0.85 b | 12.59 ± 1.32 b | 11.28 ± 0.50 b | |
| 1-methyl-4-prop-1-en-2-ylcyclohexene | 9.66 | Citric, lemon | 23.58 ± 1.67 a | 17.20 ± 1.14 c | - | - | 14.61 ± 0.71 c | 20.39 ± 0.85 b |
| 2-hydroxypropanamide | 13.84 | - | - | 10.32 ± 0.71 | - | - | - | |
| prop-2-enylurea | 19.44 | - | - | 9.75 ± 0.09 | - | - | - | |
| 1-methylpyrazole-4-carbaldehyde | 25.58 | - | - | 11.09 ± 0.42 | - | - | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavala-Díaz de la Serna, F.J.; Contreras-López, R.; Lerma-Torres, L.P.; Ruiz-Terán, F.; Rocha-Gutiérrez, B.A.; Pérez-Vega, S.B.; Elías-Ogaz, L.R.; Salmerón, I. Understanding the Biosynthetic Changes that Give Origin to the Distinctive Flavor of Sotol: Microbial Identification and Analysis of the Volatile Metabolites Profiles During Sotol (Dasylirion sp.) Must Fermentation. Biomolecules 2020, 10, 1063. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10071063
Zavala-Díaz de la Serna FJ, Contreras-López R, Lerma-Torres LP, Ruiz-Terán F, Rocha-Gutiérrez BA, Pérez-Vega SB, Elías-Ogaz LR, Salmerón I. Understanding the Biosynthetic Changes that Give Origin to the Distinctive Flavor of Sotol: Microbial Identification and Analysis of the Volatile Metabolites Profiles During Sotol (Dasylirion sp.) Must Fermentation. Biomolecules. 2020; 10(7):1063. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10071063
Chicago/Turabian StyleZavala-Díaz de la Serna, Francisco Javier, Ricardo Contreras-López, L. Paola Lerma-Torres, Francisco Ruiz-Terán, Beatriz A. Rocha-Gutiérrez, Samuel B. Pérez-Vega, Leslie R. Elías-Ogaz, and Ivan Salmerón. 2020. "Understanding the Biosynthetic Changes that Give Origin to the Distinctive Flavor of Sotol: Microbial Identification and Analysis of the Volatile Metabolites Profiles During Sotol (Dasylirion sp.) Must Fermentation" Biomolecules 10, no. 7: 1063. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10071063