Effects of the Delta Opioid Receptor Agonist DADLE in a Novel Hypoxia-Reoxygenation Model on Human and Rat-Engineered Heart Tissue: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of Rat and Human Engineered Heart Tissue (rEHT/hEHT)
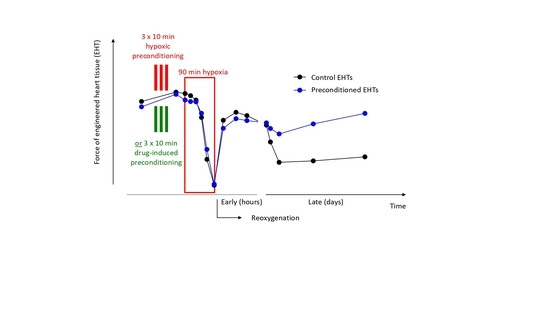
2.2. Induction of Hypoxia-Reoxygenation Injury and Hypertrophy
2.3. Mechanical Pro-Hypertrophic Intervention in EHTs
2.4. Quantification of Cellular Damage
2.5. Immunohistochemistry
2.6. Isolation of RNA and qPCR from EHTs
2.7. Statistical Analysis
3. Results
3.1. Optimization of the Experimental Procedure to Obtain a Reliable Hypoxic Trauma in EHTs
3.2. Preconditioning of EHTs by Brief Episodes of Hypoxia
3.3. Preconditioning with a δ-opioid Receptor Agonist (DADLE)
3.4. Transfer of the Hypoxia-Reoxygenation Protocol to Human EHTs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Wang, N.P.; Guyton, R.A.; Vinten-Johansen, J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am. J. Physiol Heart Circ. Physiol. 2003, 285, H579–H588. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Skyschally, A.; Walter, B.; Heusch, G. Coronary microembolization during early reperfusion: Infarct extension, but protection by ischaemic postconditioning. Eur. Heart J. 2013, 34, 3314–3321. [Google Scholar] [CrossRef]
- Davies, W.R.; Brown, A.J.; Watson, W.; McCormick, L.M.; West, N.E.; Dutka, D.P.; Hoole, S.P. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: The CRISP stent trial long-term follow-up. Circ. Cardiovasc. Interv. 2013, 6, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019. [Google Scholar] [CrossRef] [Green Version]
- Headrick, J.P.; See Hoe, L.E.; Du Toit, E.F.; Peart, J.N. Opioid receptors and cardioprotection—‘opioidergic conditioning’ of the heart. Br. J. Pharmacol. 2015, 172, 2026–2050. [Google Scholar] [CrossRef] [Green Version]
- Oeltgen, P.R.; Nilekani, S.P.; Nuchols, P.A.; Spurrier, W.A.; Su, T.P. Further studies on opioids and hibernation: Delta opioid receptor ligand selectively induced hibernation in summer-active ground squirrels. Life Sci. 1988, 43, 1565–1574. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Garcia-Dorado, D.; Bøtker, H.E.; Davidson, S.M.; Downey, J.; Engel, F.B.; Jennings, R.; Lecour, S.; Leor, J.; Madonna, R.; et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2017, 113, 564–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.; Eder, A.; Bönstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schwoerer, A.; Uebeler, J.; Eschenhagen, T. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 2010, 107, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirt, M.N.; Sörensen, N.A.; Bartholdt, L.M.; Boeddinghaus, J.; Schaaf, S.; Eder, A.; Vollert, I.; Stöhr, A.; Schulze, T.; Witten, A.; et al. Increased afterload induces pathological cardiac hypertrophy: A new in vitro model. Basic Res. Cardiol. 2012, 107, 307. [Google Scholar] [CrossRef] [Green Version]
- Breckwoldt, K.; Letuffe-Brenière, D.; Mannhardt, I.; Schulze, T.; Ulmer, B.; Werner, T.; Benzin, A.; Klampe, B.; Reinsch, C.M.; Laufer, S.; et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 2017, 12, 1177–1197. [Google Scholar] [CrossRef]
- Lecour, S.; Bøtker, H.E.; Condorelli, G.; Davidson, S.M.; Garcia-Dorado, D.; Engel, F.B.; Ferdinandy, P.; Heusch, G.; Madonna, R.; Ovize, M.; et al. ESC working group cellular biology of the heart: Position paper: Improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc. Res. 2014, 104, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.-W.; Seol, Y.-J.; Zhang, Y.S.; Shin, S.-R.; Zhao, L.; Aleman, J.; et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef]
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A.; et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell Cardiol. 2014, 74, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac tissue engineering: State of the art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulmer, B.M.; Eschenhagen, T. Human pluripotent stem cell-derived cardiomyocytes for studying energy metabolism. Biochim. Biophys. Acta Mol. Cell Res. 2019. [Google Scholar] [CrossRef]
- Görbe, A.; Eder, A.; Varga, Z.V.; Pálóczi, J.; Hansen, A.; Ferdinandy, P.; Eschenhagen, T. Protection by the NO-Donor SNAP and BNP against Hypoxia/Reoxygenation in Rat Engineered Heart Tissue. PLoS ONE 2015, 10, e0132186. [Google Scholar] [CrossRef]
- Jones, S.P.; Tang, X.L.; Guo, Y.; Steenbergen, C.; Lefer, D.J.; Kukreja, R.C.; Kong, M.; Li, Q.; Bhushan, S.; Zhu, X.; et al. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): A new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ. Res. 2015, 116, 572–586. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Vunjak-Novakovic, G. Human Tissue-Engineered Model of Myocardial Ischemia-Reperfusion Injury. Tissue Eng. Part. A 2019, 25, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Katare, R.G.; Ando, M.; Kakinuma, Y.; Sato, T. Engineered heart tissue: A novel tool to study the ischemic changes of the heart in vitro. PLoS ONE 2010, 5, e9275. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.E.; Rose, E.; Yao, Z.; Gross, G.J. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am. J. Physiol. 1995, 268, H2157–H2161. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kersten, J.R.; Riess, M.L. Opioid-induced cardioprotection. Curr. Pharm. Des. 2014, 20, 5696–5705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittert, G.; Hope, P.; Pyle, D. Tissue distribution of opioid receptor gene expression in the rat. Biochem. Biophys. Res. Commun. 1996, 218, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fuscoe, J.C.; Zhao, C.; Guo, C.; Jia, M.; Qing, T.; Bannon, D.I.; Lancashire, L.; Bao, W.; Du, T.; et al. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat. Commun. 2014, 5, 3230. [Google Scholar] [CrossRef]
- Codd, E.E.; Shank, R.P.; Schupsky, J.J.; Raffa, R.B. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: Structural determinants and role in antinociception. J. Pharmacol. Exp. Ther. 1995, 274, 1263–1270. [Google Scholar] [PubMed]
- Roehl, A.B.; Funcke, S.; Becker, M.M.; Goetzenich, A.; Bleilevens, C.; Rossaint, R.; Steendijk, P.; Hein, M. Xenon and isoflurane reduce left ventricular remodeling after myocardial infarction in the rat. Anesthesiology 2013, 118, 1385–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirt, M.N.; Werner, T.; Indenbirken, D.; Alawi, M.; Demin, P.; Kunze, A.C.; Stenzig, J.; Starbatty, J.; Hansen, A.; Fiedler, J.; et al. Deciphering the microRNA signature of pathological cardiac hypertrophy by engineered heart tissue- and sequencing-technology. J. Mol. Cell. Cardiol. 2015, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.R.; Kunze, A.C.; Stenzig, J.; Eschenhagen, T.; Hirt, M.N. Blockade of miR-140-3p prevents functional deterioration in afterload-enhanced engineered heart tissue. Sci. Rep. 2019, 9, 11494. [Google Scholar] [CrossRef]
- Längle, D.; Werner, T.R.; Wesseler, F.; Reckzeh, E.; Schaumann, N.; Drowley, L.; Polla, M.; Plowright, A.T.; Hirt, M.N.; Eschenhagen, T.; et al. Toward Second-Generation Cardiomyogenic and Anti-cardiofibrotic 1,4-Dihydropyridine-Class TGFbeta Inhibitors. ChemMedChem 2019, 14, 810–822. [Google Scholar] [CrossRef] [Green Version]
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Brenière, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schulze, T.; et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Rep. 2016, 7, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Ulmer, B.M.; Stoehr, A.; Schulze, M.L.; Patel, S.; Gucek, M.; Mannhardt, I.; Funcke, S.; Murphy, E.; Eschenhagen, T.; Hansen, A. Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes. Stem Cell Rep. 2018, 10, 834–847. [Google Scholar] [CrossRef] [Green Version]
- Bell, S.P.; Sack, M.N.; Patel, A.; Opie, L.H.; Yellon, D.M. Delta opioid receptor stimulation mimics ischemic preconditioning in human heart muscle. J. Am. Coll. Cardiol. 2000, 36, 2296–2302. [Google Scholar] [CrossRef] [Green Version]
- Fuardo, M.; Lemoine, S.; Lo Coco, C.; Hanouz, J.L.; Massetti, M. [D-Ala2,D-Leu5]-enkephalin (DADLE) and morphine-induced postconditioning by inhibition of mitochondrial permeability transition pore, in human myocardium. Exp. Biol. Med. (Maywood) 2013, 238, 426–432. [Google Scholar] [CrossRef]
- Huang, M.-H.; Wang, H.-Q.; Roeske, W.R.; Birnbaum, Y.; Wu, Y.; Yang, N.-P.; Lin, Y.; Ye, Y.; McAdoo, D.J.; Hughes, M.G.; et al. Mediating delta-opioid-initiated heart protection via the beta2-adrenergic receptor: Role of the intrinsic cardiac adrenergic cell. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H376–H384. [Google Scholar] [CrossRef] [Green Version]
- Sobanski, P.; Krajnik, M.; Shaqura, M.; Bloch-Boguslawska, E.; Schafer, M.; Mousa, S.A. The presence of mu-, delta-, and kappa-opioid receptors in human heart tissue. Heart Vessels 2014, 29, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, U.; Müller, C.; Röcken, C.; Laube, B.; Täger, M.; Huth, C.; Klein, H.U.; Goette, A. Expression of opioid receptor subtypes and their ligands in fibrillating human atria. Pacing Clin. Electrophysiol. 2005, 28, S275–S279. [Google Scholar] [CrossRef] [PubMed]
- Lemme, M.; Ulmer, B.M.; Lemoine, M.D.; Zech, A.T.L.; Flenner, F.; Ravens, U.; Reichenspurner, H.; Rol-Garcia, M.; Smith, G.; Hansen, A.; et al. Atrial-like Engineered Heart Tissue: An In Vitro Model of the Human Atrium. Stem Cell Rep. 2018, 11, 1378–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.S.; Richards, S.C.; Olsson, R.A.; Mullane, K.; Walsh, R.S.; Downey, J.M. Evidence that the adenosine A3 receptor may mediate the protection afforded by preconditioning in the isolated rabbit heart. Cardiovasc. Res. 1994, 28, 1057–1061. [Google Scholar] [CrossRef]
- Stadler, B.; Phillips, J.; Toyoda, Y.; Federman, M.; Levitsky, S.; McCully, J.D. Adenosine-enhanced ischemic preconditioning modulates necrosis and apoptosis: Effects of stunning and ischemia-reperfusion. Ann. Thorac. Surg. 2001, 72, 555–563. [Google Scholar] [CrossRef]
- Walker, D.M.; Walker, J.M.; Pugsley, W.B.; Pattison, C.W.; Yellon, D.M. Preconditioning in isolated superfused human muscle. J. Mol. Cell. Cardiol. 1995, 27, 1349–1357. [Google Scholar] [CrossRef]
- Li, Y.; Kloner, R.A. The cardioprotective effects of ischemic ’preconditioning’ are not mediated by adenosine receptors in rat hearts. Circulation 1993, 87, 1642–1648. [Google Scholar] [CrossRef] [Green Version]
- Gross, G.J.; Hsu, A.; Nithipatikom, K.; Bobrova, I.; Bissessar, E. Eribis peptide 94 reduces infarct size in rat hearts via activation of centrally located mu opioid receptors. J. Cardiovasc. Pharmacol. 2012, 59, 194–197. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funcke, S.; Werner, T.R.; Hein, M.; Ulmer, B.M.; Hansen, A.; Eschenhagen, T.; Hirt, M.N. Effects of the Delta Opioid Receptor Agonist DADLE in a Novel Hypoxia-Reoxygenation Model on Human and Rat-Engineered Heart Tissue: A Pilot Study. Biomolecules 2020, 10, 1309. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10091309
Funcke S, Werner TR, Hein M, Ulmer BM, Hansen A, Eschenhagen T, Hirt MN. Effects of the Delta Opioid Receptor Agonist DADLE in a Novel Hypoxia-Reoxygenation Model on Human and Rat-Engineered Heart Tissue: A Pilot Study. Biomolecules. 2020; 10(9):1309. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10091309
Chicago/Turabian StyleFuncke, Sandra, Tessa R. Werner, Marc Hein, Bärbel M. Ulmer, Arne Hansen, Thomas Eschenhagen, and Marc N. Hirt. 2020. "Effects of the Delta Opioid Receptor Agonist DADLE in a Novel Hypoxia-Reoxygenation Model on Human and Rat-Engineered Heart Tissue: A Pilot Study" Biomolecules 10, no. 9: 1309. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10091309