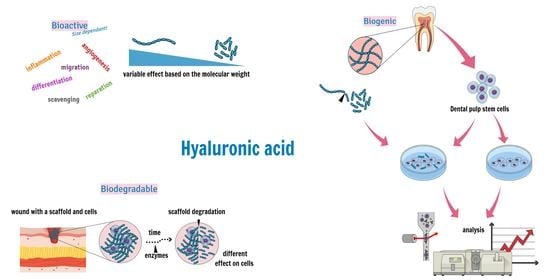
Low Molecular Weight Hyaluronic Acid Effect on Dental Pulp Stem Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hyaluronic Acid
2.2. Tooth Collection
2.3. Dental Pulp Isolation
2.4. Cell Mobilisation, Expansion, and Banking
2.5. Cell Cultivation
2.6. Cell Characteristics
2.7. Statistical Analysis
3. Results
3.1. Cell Viability
3.2. Cell Size
3.3. Proliferation Activity
3.4. Surface Markers Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Battistini, F.D.; Tártara, L.I.; Boiero, C.; Guzman, M.L.; Luciani-Giaccobbe, L.; Palma, S.D.; Allemandi, D.A.; Manzo, R.H.; Olivera, M.E. The role of hyaluronan as a drug carrier to enhance the bioavailability of extended release ophthalmic formulations. Hyaluronan-timolol ionic complexes as a model case. Eur. J. Pharm. Sci. 2017, 105, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Cowman, M.K.; Lee, H.G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol. 2015, 6, 261. [Google Scholar] [CrossRef] [Green Version]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Albano, G.D.; Bonanno, A.; Cavalieri, L.; Ingrassia, E.; Di Sano, C.; Siena, L.; Riccobono, L.; Gagliardo, R.; Profita, M. Effect of High, Medium, and Low Molecular Weight Hyaluronan on Inflammation and Oxidative Stress in an In Vitro Model of Human Nasal Epithelial Cells. Mediators Inflamm. 2016, 2016, 8727289. [Google Scholar] [CrossRef] [Green Version]
- Kogan, G.; Šoltés, L.; Stern, R.; Schiller, J.; Mendichi, R. Hyaluronic Acid: Its Function and Degradation in in vivo Systems. Stud. Nat. Prod. Chem. 2008, 34, 789–882. [Google Scholar] [CrossRef]
- Frenkel, J.S. The role of hyaluronan in wound healing. Int. Wound J. 2014, 11, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell. Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyman, E.; Henricson, J.; Ghafouri, B.; Anderson, C.D.; Kratz, G. Hyaluronic Acid Accelerates Re-epithelialization and Alters Protein Expression in a Human Wound Model. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2221. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, Y.; Kim, H.; Kim, K.; Lee, Y.-S.; Choe, J.; Hahn, J.-H.; Lee, H.; Jeon, J.; Choi, C. Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGFβ receptor interaction via CD44-PKCδ. Mol. Cells 2012, 33, 563–574. [Google Scholar] [CrossRef]
- Wu, M.; Cao, M.; He, Y.; Liu, Y.; Yang, C.; Du, Y.; Wang, W.; Gao, F. A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J. 2015, 29, 1290–1298. [Google Scholar] [CrossRef]
- Kultti, A.; Li, X.; Jiang, P.; Thompson, C.B.; Frost, G.I.; Shepard, H.M. Therapeutic targeting of hyaluronan in the tumor stroma. Cancers 2012, 4, 873–903. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, J.B.; El-Ashry, D.; Turley, E.A. Hyaluronan, cancer-associated fibroblasts and the tumor microenvironment in malignant progression. Front. Cell Dev. Biol. 2018, 6, 48. [Google Scholar] [CrossRef]
- Evanko, S.P.; Potter-Perigo, S.; Petty, L.J.; Workman, G.A.; Wight, T.N. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-β1 induction of lung myofibroblasts. Matrix Biol. 2015, 42, 74–92. [Google Scholar] [CrossRef] [Green Version]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [Green Version]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, A.S.; Pilling, D.; Gomer, R.H. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS ONE 2011, 6, e26078. [Google Scholar] [CrossRef] [PubMed]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Wang, Y.-M.; Yang, J.; Luo, X.-S. Hyaluronic acid: A versatile biomaterial in tissue engineering. J. Plast. Aesthet. Res. 2017, 4, 219. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Luttrell, T.; Rosenberry, S.; Estacado, N.; Coates, J. Novel Use of a Biologically Active-Prefabricated-Random-Three-Dimensional-Polymer Scaffold of Hyaluronic Acid (HYAFF) to Facilitate Complicated Wound Closure. In Burns, Infections and Wound Management; Shiffman, M.A., Low, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 213–247. [Google Scholar]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic acid–Based activatable nanomaterials for stimuli-responsive imaging and therapeutics: Beyond CD44-mediated drug delivery. J. Adv. Mater. 2019, 31, 1803549. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic Acid-Based Nanomaterials for Cancer Therapy. Polymers 2018, 10, 1133. [Google Scholar] [CrossRef] [Green Version]
- Murashita, T.; Nakayama, Y.; Hirano, T.; Ohashi, S. Acceleration of granulation tissue ingrowth by hyaluronic acid in artificial skin. Br. J. Plast. Surg. 1996, 49, 58–63. [Google Scholar] [CrossRef]
- Li, H.; Qi, Z.; Zheng, S.; Chang, Y.; Kong, W.; Fu, C.; Yu, Z.; Yang, X.; Pan, S. The application of hyaluronic acid-based hydrogels in bone and cartilage tissue engineering. Adv. Eng. Mater. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [Green Version]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 1908996. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Sakai, N.; Shiba, H.; Nagahara, T.; Fujita, T.; Kajiya, M.; Iwata, T.; Matsuda, S.; Kawahara, K.; Kawaguchi, H. Characteristics of high-molecular-weight hyaluronic acid as a brain-derived neurotrophic factor scaffold in periodontal tissue regeneration. Tissue Eng. Part A 2011, 17, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Basu, P.; Sukul, M.; Mano, J.F.; Reseland, J.E. Injectable Biomaterials for Dental Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3442. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.N.; Khalilov, R.; Samiei, M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int. J. Biol. Macromol. 2019, 140, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable hydrogel with slow degradability composed of gelatin and hyaluronic acid cross-linked by schiff’s base formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef]
- Heljak, M.K.; Swieszkowski, W.; Kurzydlowski, K.J. Modeling of the degradation kinetics of biodegradable scaffolds: The effects of the environmental conditions. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhang, W. Control of scaffold degradation in tissue engineering: A review. Tissue Eng. Part. B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Hortensius, R.A.; Harley, B.A.C. 2.16 Collagen-GAG Materials. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; Volume 2, pp. 351–380. [Google Scholar]
- Martins, J.P.; Ferreira, M.P.A.; Ezazi, N.Z.; Hirvonen, J.T.; Santos, H.A.; Thrivikraman, G.; França, C.M.; Athirasala, A.; Tahayeri, A.; Bertassoni, L.E. 3D printing: Prospects and challenges. In Nanotechnologies in Preventive and Regenerative Medicine; Uskoković, V., Uskoković, D.P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2018; pp. 299–379. [Google Scholar]
- Victor, A.K.; Reiter, L.T. Dental pulp stem cells for the study of neurogenetic disorders. Hum. Mol. Genet. 2017, 26, R166–R171. [Google Scholar] [CrossRef] [Green Version]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef]
- Suchánek, J.; Browne, K.Z.; Kleplová, T.S.; Mazurová, Y. Protocols for Dental-Related Stem Cells Isolation, Amplification and Differentiation. In Dental Stem Cells: Regenerative Potential; Humana Press: Totowa, NJ, USA, 2016; pp. 27–56. [Google Scholar]
- Tsutsui, T.W. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning Adv. Appl. 2020, 13, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Luzuriaga, J.; Pastor-Alonso, O.; Encinas, J.M.; Unda, F.; Ibarretxe, G.; Pineda, J.R. Human dental pulp stem cells grown in neurogenic media differentiate into endothelial cells and promote neovasculogenesis in the mouse brain. Front. Physiol. 2019, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Goorha, S.; Reiter, L.T. Culturing and Neuronal Differentiation of Human Dental Pulp Stem Cells. Curr. Protoc. Hum. Genet. 2017, 92, 21.6.1–21.6.10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, T.G.; Paque, F.; Sven Patyna, M.; Willershausen, B.; Briseno-Marroquin, B. Three-dimensional analysis of the physiological foramen geometry of maxillary and mandibular molars by means of micro-CT. Int. J. Oral Sci. 2017, 9, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, M.; Hirata, A. The dental pulp: Composition, properties and functions. JSM Dent. 2017, 5, 1079. [Google Scholar]
- Niloy, K.K.; Gulfam, M.; Compton, K.B.; Li, D.; Huang, G.T.-J.; Lowe, T.L. Methacrylated Hyaluronic Acid–Based Hydrogels Maintain Stemness in Human Dental Pulp Stem Cells. Regen. Eng. Transl. Med. 2019, 1–11. [Google Scholar] [CrossRef]
- Compton, K.B. Synthesis and Characterization of Methacrylated Hyaluronan-Based Hydrogels for Tissue Engineering. Master’s Thesis, University of Tennessee, Memphis, TN, USA, December 2014. [Google Scholar]
- Jones, T.D.; Kefi, A.; Sun, S.; Cho, M.; Alapati, S.B. An Optimized Injectable Hydrogel Scaffold Supports Human Dental Pulp Stem Cell Viability and Spreading. Adv. Med. 2016, 2016, 7363579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, L.; Babo, P.; Hebling, J.; Gomes, M.; Reis, R. Injectable photocrosslinkable hyaluronic acid hydrogels incorporated with platelet lysate enhance the dentinogenic differentiation of human dental pulp stem cells. In Proceedings of the 10th World Biomaterials Congress, Montréal, QC, Canada, 17 May–22 May 2016. [Google Scholar]
- Umemura, N.; Ohkoshi, E.; Tajima, M.; Kikuchi, H.; Katayama, T.; Sakagami, H. Hyaluronan induces odontoblastic differentiation of dental pulp stem cells via CD44. Stem Cell Res. Ther. 2016, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.; Kraft, D.C.; Lysdahl, H.; Foldager, C.B.; Chen, M.; Kristiansen, A.A.; Rolfing, J.H.; Bunger, C.E. Functionalization of polycaprolactone scaffolds with hyaluronic acid and beta-TCP facilitates migration and osteogenic differentiation of human dental pulp stem cells in vitro. Tissue Eng. Part A 2015, 21, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Apel, C.; Buttler, P.; Salber, J.; Dhanasingh, A.; Neuss, S. Differential mineralization of human dental pulp stem cells on diverse polymers. Biomed. Tech. 2018, 63, 261–269. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Sivolella, S.; Brunello, G.; Berengo, M.; Piattelli, A.; Bressan, E.; Zavan, B. A hyaluronan-based scaffold for the in vitro construction of dental pulp-like tissue. Int. J. Mol. Sci. 2015, 16, 4666–4681. [Google Scholar] [CrossRef] [Green Version]
- Chrepa, V.; Austah, O.; Diogenes, A. Evaluation of a commercially available hyaluronic acid hydrogel (Restylane) as injectable scaffold for dental pulp regeneration: An in vitro evaluation. J. Endod. 2017, 43, 257–262. [Google Scholar] [CrossRef]
- Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V.; Kubala, L. Absence of differences among low, middle, and high molecular weight hyaluronan in activating murine immune cells in vitro. Int. J. Biol. Macromol. 2018, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessio, N.; Stellavato, A.; Squillaro, T.; Del Gaudio, S.; Di Bernardo, G.; Peluso, G.; De Rosa, M.; Schiraldi, C.; Galderisi, U. Hybrid complexes of high and low molecular weight hyaluronan delay in vitro replicative senescence of mesenchymal stromal cells: A pilot study for future therapeutic application. Aging 2018, 10, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.E.; Li, C.; Garland, J.M.; Kumar, S. CD105 is important for angiogenesis: Evidence and potential applications. FASEB J. 2003, 17, 984–992. [Google Scholar] [CrossRef]
- Brinkhof, B.; Zhang, B.; Cui, Z.; Ye, H.; Wang, H. ALCAM (CD166) as a gene expression marker for human mesenchymal stromal cell characterisation. Gene X 2020, 5, 100031. [Google Scholar] [CrossRef]
- Yang, Z.X.; Han, Z.B.; Ji, Y.R.; Wang, Y.W.; Liang, L.; Chi, Y.; Yang, S.G.; Li, L.N.; Luo, W.F.; Li, J.P.; et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS ONE 2013, 8, e59354. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Nicolau, N.; Raquel, M.; Fariñas, O.; Savio, A.; Vilarrodona, A.; Casaroli-Marano, R.P. Extrinsic modulation of integrin α6 and progenitor cell behavior in mesenchymal stem cells. Stem Cell Res. 2020, 47, 101899. [Google Scholar] [CrossRef]
- Foster, B.M.; Zaidi, D.; Young, T.R.; Mobley, M.E.; Kerr, B.A. CD117/c-kit in Cancer Stem Cell-Mediated Progression and Therapeutic Resistance. Biomedicines 2018, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Edling, C.E.; Hallberg, B. c-Kit—a hematopoietic cell essential receptor tyrosine kinase. Int. J. Biochem. Cell Biol. 2007, 39, 1995–1998. [Google Scholar] [CrossRef]
- Crende, O.; García-Gallastegui, P.; Luzuriaga, J.; Badiola, I.; de la Hoz, C.; Unda, F.; Ibarretxe, G.; Pineda, J. Is There Such a Thing as a Genuine Cancer Stem Cell Marker? Perspectives from the Gut, the Brain and the Dental Pulp. Biology 2020, 9, 426. [Google Scholar] [CrossRef]
- Espagnolle, N.; Guilloton, F.; Deschaseaux, F.; Gadelorge, M.; Sensébé, L.; Bourin, P. CD 146 expression on mesenchymal stem cells is associated with their vascular smooth muscle commitment. J. Cell. Mol. Med. 2014, 18, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Kobayashi, T.; Tsutsui, T.W. CD146 positive human dental pulp stem cells promote regeneration of dentin/pulp-like structures. Hum. Cell 2018, 31, 127–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Ling, J.; Wu, L.; Liu, L.; Xiao, Y. Expression of mineralization markers in dental pulp cells. J. Endod. 2007, 33, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Khanna-Jain, R.; Vanhatupa, S.; Vuorinen, A.; Sandor, G.; Suuronen, R.; Mannerstrom, B.; Miettinen, S. Growth and differentiation of human dental pulp stem cells maintained in fetal bovine serum, human serum and serum-free/xeno-free culture media. Stem Cell Res. Ther. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Karbanová, J.; Soukup, T.; Suchánek, J.; Pytlík, R.; Corbeil, D.; Mokrý, J. Characterization of Dental Pulp Stem Cells from Impacted Third Molars Cultured in Low Serum-Containing Medium. Cells Tissues Organs 2011, 193, 344–365. [Google Scholar] [CrossRef] [Green Version]
- Nam, H.; Kim, G.-H.; Bae, Y.-K.; Jeong, D.-E.; Joo, K.-M.; Lee, K.; Lee, S.-H. Angiogenic capacity of dental pulp stem cell regulated by SDF-1α-CXCR4 axis. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef]
- Young, F.I.; Telezhkin, V.; Youde, S.J.; Langley, M.S.; Stack, M.; Kemp, P.J.; Waddington, R.J.; Sloan, A.J.; Song, B. Clonal Heterogeneity in the Neuronal and Glial Differentiation of Dental Pulp Stem/Progenitor Cells. Stem Cells Int. 2016, 2016, 1290561. [Google Scholar] [CrossRef] [Green Version]
- Alraies, A.; Canetta, E.; Waddington, R.J.; Moseley, R.; Sloan, A.J. Discrimination of Dental Pulp Stem Cell Regenerative Heterogeneity by Single-Cell Raman Spectroscopy. Tissue Eng. Part C Methods 2019, 25, 489–499. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Zhong, L.; You, M.; Yan, Z.; Luo, M.; Zhang, B.; Yang, B.; Chen, Q. Homogeneity and heterogeneity of biological characteristics in mesenchymal stem cells from human umbilical cords and exfoliated deciduous teeth. Int. J. Biochem. Cell Biol. 2020, 98, 415–425. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Alraies, A.; Alaidaroos, N.Y.; Waddington, R.J.; Moseley, R.; Sloan, A.J. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol. 2017, 18, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Viejo, M.; Menéndez-Menéndez, Y.; Otero-Hernández, J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World. J. Stem Cells 2015, 7, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Lee, H.L.; Hong, C.; Wang, C.Y. Single CD271 marker isolates mesenchymal stem cells from human dental pulp. Int. J. Oral Sci. 2015, 7, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Mafi, P.; Hindocha, S.; Mafi, R.; Griffin, M.; Khan, W. Adult mesenchymal stem cells and cell surface characterization—a systematic review of the literature. Open J. Orthop. 2011, 5, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrin, D.; Alghadeer, A.; Zhao, Y.T.; Miklas, J.W.; Hussein, A.M.; Detraux, D.; Robitaille, A.M.; Madan, A.; Moon, R.T.; Wang, Y.J.S.R. Metabolism as an early predictor of DPSCs Aging. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]





| Cell Line | Sex | Age | Tooth Specification |
|---|---|---|---|
| Line 1 | Female | 14 | Lower third molar |
| Line 2 | Female | 15 | Lower third molar |
| Line 3 | Male | 13 | Upper second premolar |
| Line 4 | Female | 18 | Lower third molar |
| Line 5 | Female | 15 | Lower third molar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, J.; Pilbauerova, N.; Soukup, T.; Suchankova-Kleplova, T.; Suchanek, J. Low Molecular Weight Hyaluronic Acid Effect on Dental Pulp Stem Cells In Vitro. Biomolecules 2021, 11, 22. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010022
Schmidt J, Pilbauerova N, Soukup T, Suchankova-Kleplova T, Suchanek J. Low Molecular Weight Hyaluronic Acid Effect on Dental Pulp Stem Cells In Vitro. Biomolecules. 2021; 11(1):22. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010022
Chicago/Turabian StyleSchmidt, Jan, Nela Pilbauerova, Tomas Soukup, Tereza Suchankova-Kleplova, and Jakub Suchanek. 2021. "Low Molecular Weight Hyaluronic Acid Effect on Dental Pulp Stem Cells In Vitro" Biomolecules 11, no. 1: 22. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010022