Plasticity-Related Activity in the Hippocampus, Anterior Cingulate, Orbitofrontal, and Prefrontal Cortex Following a Repeated Treatment with D2/D3 Agonist Quinpirole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
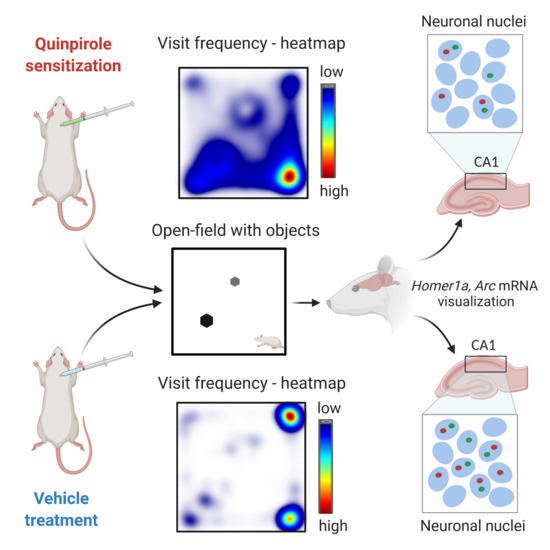
2.2. Drug Administration
2.3. The Apparatus: Object-Enriched Arena
2.4. Behavioral Procedure
2.5. Behavioral Analysis and Statistics
2.6. Tissue Preparation
2.7. Image Acquisition
2.8. Image Analysis
2.9. Similarity Score—A Measure of IEG Overlap between A1 and A2
2.10. Statistical Analysis
3. Results
3.1. Locomotor Sensitization Induced by QNP Treatment
3.2. Stereotypical Checking—Experimental Days 1–10
3.3. Stereotypical Checking in Sessions A1 and A2 (Experimental Day 11)
3.4. IEG Expression in the Hippocampus, OFC, ACC, and mPFC
3.5. Overlap of IEG Expressing Neurons between A1 and A2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Lopez, A.D. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2010; Harvard University Press: Cambridge, MA, USA, 1996; Volume 1, pp. 1–35. [Google Scholar]
- Swain, S.; Behura, S.A. Comparative study of quality of life and disability among schizophrenia and obsessive-compulsive dis-order patients in remission. Ind. Psychiatry J. 2016, 25, 210. [Google Scholar]
- Albert, U.; Marazziti, D.; Di Salvo, G.; Solia, F.; Rosso, G.; Maina, G. A Systematic Review of Evidence-based Treatment Strategies for Obsessive- compulsive Disorder Resistant to first-line Pharmacotherapy. Curr. Med. Chem. 2017, 25, 5647–5661. [Google Scholar] [CrossRef]
- Hirschtritt, M.E.; Bloch, M.H.; Mathews, C.A. Obsessive-compulsive disorder advances in diagnosis and treatment. JAMA J. Am. Med. Assoc. 2017, 317, 1358–1367. [Google Scholar] [CrossRef]
- Bloch, M.H.; Green, C.; Kichuk, S.A.; Dombrowski, P.A.; Wasylink, S.; Billingslea, E.; Landeros-Weisenberger, A.; Kelmendi, B.; Goodman, W.K.; Leckman, J.F.; et al. Long-term outcome in adults with obses-sive-compulsive disorder. Depress. Anxiety 2013, 30, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Baxter, L.R.; Schwartz, J.M.; Mazziotta, J.C.; Phelps, M.E.; Pahl, J.J.; Guze, B.H.; Fairbanks, L. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am. J. Psychiatry 1988, 145, 1560–1563. [Google Scholar] [CrossRef]
- Perani, D.; Colombo, C.; Bressi, S.; Bonfanti, A.; Grassi, F.; Scarone, S.; Bellodi, L.; Smeraldi, E.; Fazio, F. [18F]FDG PET Study in Obsessive–Compulsive Disorder: A Clinical/Metabolic Correlation Study after Treatment. Br. J. Psychiatry 1995, 166, 244–250. [Google Scholar] [CrossRef]
- Del Casale, A.; Kotzalidis, G.D.; Rapinesi, C.; Serata, D.; Ambrosi, E.; Simonetti, A.; Pompili, M.; Ferracuti, S.; Tatarelli, R.; Girardi, P. Functional Neuroimaging in Obsessive-Compulsive Disorder. Neuropsychobiology 2011, 64, 61–85. [Google Scholar] [CrossRef]
- Milad, M.R.; Rauch, S.L. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 2012, 16, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.S.; Shin, Y.-W.; Kim, C.-W.; Kim, Y.I.; Youn, T.; Han, M.H.; Chang, K.-H.; Kim, J.-J. Similarity and disparity of obsessive-compulsive disorder and schizophrenia in MR volumetric abnormalities of the hippocampus-amygdala complex. J. Neurol. Neurosurg. Psychiatry 2003, 74, 962–964. [Google Scholar] [CrossRef]
- Göttlich, M.; Krämer, U.M.; Kordon, A.; Hohagen, F.; Zurowski, B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum. Brain Mapp. 2014, 35, 5617–5632. [Google Scholar] [CrossRef]
- Adler, C.M.; McDonough-Ryan, P.; Sax, K.W.; Holland, S.K.; Arndt, S.; Strakowski, S.M. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J. Psychiatr. Res. 2000, 34, 317–324. [Google Scholar] [CrossRef]
- Marsh, R.; Tau, G.Z.; Wang, Z.; Huo, Y.; Liu, G.; Hao, X.; Packard, M.G.; Peterson, B.S.; Simpson, H.B. Reward-based spatial learning in unmedicated adults with obsessive-compulsive disorder. Am. J. Psychiatry 2015, 172, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, S.L.; Savage, C.R.; Alpert, N.M.; Dougherty, D.; Kendrick, A.; Curran, T.; Brown, H.D.; Manzo, P.; Fischman, A.J.; Jenike, M.A. Probing striatal function in obsessive-compulsive disorder: A PET study of implicit sequence learning. J. Neuropsychiatry Clin. Neurosci. 1997, 9, 568–573. [Google Scholar] [PubMed] [Green Version]
- Kang, D.H.; Kwon, J.S.; Kim, J.J.; Youn, T.; Park, H.J.; Kim, M.S.; Lee, D.S.; Lee, M.C. Brain glucose metabolic changes associated with neuropsy-chological improvements after 4 months of treatment in patients with obsessive-compulsive disorder. Acta Psychiatr. Scand 2003, 107, 291–297. [Google Scholar] [CrossRef]
- Atmaca, M.; Yildirim, H.; Ozdemir, H.; Koc, M.; Ozler, S.; Tezcan, E. Neurochemistry of the hippocampus in patients with obsessive-compulsive disorder: Regular article. Psychiatry Clin. Neurosci. 2009, 63, 486–490. [Google Scholar] [CrossRef]
- Atmaca, M.; Yildirim, H.; Ozdemir, H.; Ozler, S.; Kara, B.; Ozler, Z.; Kanmaz, E.; Mermi, O.; Tezcan, E. Hippocampus and amygdalar volumes in patients with refractory obsessive–compulsive disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2008, 32, 1283–1286. [Google Scholar] [CrossRef]
- Reess, T.J.; Rus, O.G.; Gürsel, D.A.; Schmitz-Koep, B.; Wagner, G.; Berberich, G.; Koch, K. Association between hippocampus volume and symptom profiles in obsessive-compulsive disorder. NeuroImage Clin. 2018, 17, 474–480. [Google Scholar] [CrossRef]
- Heuvel, O.A.V.D.; Boedhoe, P.S.; Bertolin, S.; Bruin, W.B.; Francks, C.; Ivanov, I.; Jahanshad, N.; Kong, X.; Kwon, J.S.; O’Neill, J.; et al. An overview of the first 5 years of the ENIGMA obsessive–compulsive disorder working group: The power of worldwide collaboration. Hum. Brain Mapp. 2020. [Google Scholar] [CrossRef] [Green Version]
- Kettl, P.A.; Marks, I.M. Neurological Factors in Obsessive Compulsive Disorder: Two case reports and a review of the literature. Br. J. Psychiatry 1986, 149, 315–319. [Google Scholar] [CrossRef]
- Stern, T.A.; Murray, G.B. Complex Partial Seizures Presenting as a Psychiatric Illness. J. Nerv. Ment. Dis. 1984, 172, 625–627. [Google Scholar] [CrossRef]
- Max, J.E.; Smith, W.L.; Lindgren, S.D.; Robin, N.A.; Mattheis, P.; Stierwalt, J.; Morrisey, M. Case Study: Obsessive-Compulsive Disorder after Severe Traumatic Brain Injury in an Adolescent. J. Am. Acad. Child Adolesc. Psychiatry 1995, 34, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, K.L.; Philbeck, J.W.; Barr, W.B.; Devinsky, O.; Alper, K. Obsessive-compulsive symptoms in patients with temporal lobe epi-lepsy. Epilepsy Behav. 2004, 5, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, P.W. Obsessive–compulsive disorder in chronic epilepsy. Epilepsy Behav. 2011, 22, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szechtman, H.; Eckert, M.J.; Tse, W.S.; Boersma, J.T.; Bonura, C.A.; McClelland, J.Z.; Culver, K.E.; Eilam, D. Compulsive checking behavior of quinpirole-sensitized rats as an animal model of Obsessive-Compulsive Disorder(OCD): Form and control. BMC Neurosci. 2001, 2, 4. [Google Scholar] [CrossRef]
- Hatalova, H.; Radostová, D.; Pistikova, A.; Vales, K.; Stuchlik, A. Spatial Reversal Learning in Chronically Sensitized Rats and in Undrugged Sensitized Rats with Dopamine D2-Like Receptor Agonist Quinpirole. Front. Behav. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Hatalova, H.; Radostova, D.; Pistikova, A.; Vales, K.; Stuchlík, A. Detrimental effect of clomipramine on hippocampus-dependent learning in an animal model of obsessive-compulsive disorder induced by sensitization with d2/d3 agonist quinpirole. Behav. Brain Res. 2017, 317, 210–217. [Google Scholar] [CrossRef]
- Chawla, M.K.; Guzowski, J.F.; Ramirez-Amaya, V.; Lipa, P.; Hoffman, K.L.; Marriott, L.K.; Worley, P.F.; McNaughton, B.L.; Barnes, C.A. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial ex-perience. Hippocampus 2005, 15, 579–586. [Google Scholar] [CrossRef]
- Bramham, C.R.; Worley, P.F.; Moore, M.J.; Guzowski, J.F. The Immediate Early Gene Arc/Arg3.1: Regulation, Mechanisms, and Function. J. Neurosci. 2008, 28, 11760–11767. [Google Scholar] [CrossRef]
- Ramirez-Amaya, V.; Angulo-Perkins, A.; Chawla, M.K.; Barnes, C.A.; Rosi, S. Sustained Transcription of the Immediate Early Gene Arc in the Dentate Gyrus after Spatial Exploration. J. Neurosci. 2013, 33, 1631–1639. [Google Scholar] [CrossRef]
- Vazdarjanova, A.; Ramirez-Amaya, V.; Insel, N.; Plummer, T.K.; Rosi, S.; Chowdhury, S.; Mikhael, D.; Worley, P.F.; Guzowski, J.F.; Barnes, C.A. Spatial exploration inducesARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J. Comp. Neurol. 2006, 498, 317–329. [Google Scholar] [CrossRef]
- Kubik, S.; Miyashita, T.; Guzowski, J.F. Using immediate-early genes to map hippocampal subregional functions. Learn. Mem. 2007, 14, 758–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biologi-cal-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Vazdarjanova, A.; Guzowski, J.F. Differences in Hippocampal Neuronal Population Responses to Modifications of an Envi-ronmental Context: Evidence for Distinct, Yet Complementary, Functions of CA3 and CA1 Ensembles. J. Neurosci. 2004, 24, 6489–6496. [Google Scholar] [CrossRef] [Green Version]
- Swedo, S.E.; Rapoport, J.L.; Leonard, H.; Lenane, M.; Cheslow, D. Obsessive-Compulsive Disorder in Children and Adolescents. Arch. Gen. Psychiatry 1989, 46, 335. [Google Scholar] [CrossRef] [PubMed]
- Servaes, S.; Glorie, D.; Verhaeghe, J.; Wyffels, L.; Stroobants, S.; Staelens, S. [18F]-FDG PET neuroimaging in rats with quinpirole-induced checking behavior as a model for obsessive compulsive disorder. Psychiatry Res. Neuroimaging 2016, 257, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.L.; Pazdernik, T.L.; Levant, B. Differences in quinpirole-induced local cerebral glucose utilization between naive and sensitized rats. Brain Res. 2003, 964, 295–301. [Google Scholar] [CrossRef]
- Tanaka, K.Z.; He, H.; Tomar, A.; Niisato, K.; Huang, A.J.Y.; McHugh, T.J. The hippocampal engram maps experience but not place. Science 2018, 361, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Woods, C.M.; Vevea, J.L.; Chambless, D.L.; Bayen, U.J. Are Compulsive Checkers Impaired in Memory? A Meta-Analytic Review. Clin. Psychol. Sci. Pract. 2006, 9, 353–366. [Google Scholar] [CrossRef]
- Radomsky, A.S.; Dugas, M.J.; Alcolado, G.M.; Lavoie, S.L. When more is less: Doubt, repetition, memory, metamemory, and com-pulsive checking in OCD. Behav. Res. Ther. 2014, 59, 30–39. [Google Scholar] [CrossRef]
- Asaoka, N.; Nishitani, N.; Kinoshita, H.; Nagai, Y.; Hatakama, H.; Nagayasu, K.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. An Adenosine A2A Receptor Antagonist Improves Multiple Symptoms of Repeated Quinpirole-Induced Psychosis. eNeuro 2019, 6, 0366-18.2019. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brozka, H.; Alexova, D.; Radostova, D.; Janikova, M.; Krajcovic, B.; Kubík, Š.; Svoboda, J.; Stuchlik, A. Plasticity-Related Activity in the Hippocampus, Anterior Cingulate, Orbitofrontal, and Prefrontal Cortex Following a Repeated Treatment with D2/D3 Agonist Quinpirole. Biomolecules 2021, 11, 84. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010084
Brozka H, Alexova D, Radostova D, Janikova M, Krajcovic B, Kubík Š, Svoboda J, Stuchlik A. Plasticity-Related Activity in the Hippocampus, Anterior Cingulate, Orbitofrontal, and Prefrontal Cortex Following a Repeated Treatment with D2/D3 Agonist Quinpirole. Biomolecules. 2021; 11(1):84. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010084
Chicago/Turabian StyleBrozka, Hana, Daniela Alexova, Dominika Radostova, Martina Janikova, Branislav Krajcovic, Štěpán Kubík, Jan Svoboda, and Ales Stuchlik. 2021. "Plasticity-Related Activity in the Hippocampus, Anterior Cingulate, Orbitofrontal, and Prefrontal Cortex Following a Repeated Treatment with D2/D3 Agonist Quinpirole" Biomolecules 11, no. 1: 84. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11010084