Modulation of Glycinergic Neurotransmission may Contribute to the Analgesic Effects of Propacetamol
Abstract
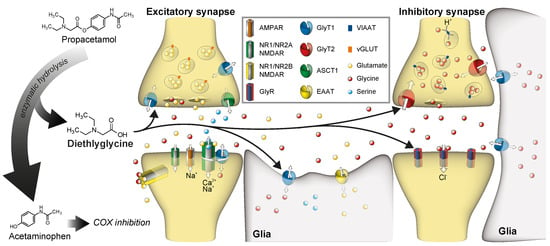
:1. Introduction
2. Materials and Methods
2.1. Drugs and Substances
2.2. cRNA-Synthesis and Microinjection
2.3. Perfusion Regime
2.4. Electrophysiology
3. Results
3.1. Functional Characterization of GlyTs
3.2. GlyT Responses to Propacetamol and Paracetamol and DEG
3.3. Characterization of the Metabolite DEG
3.4. DEG Influence on GlyR Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of Chronic Pain in Europe: Prevalence, Impact on Daily Life, and Treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Brix Finnerup, N.; Hein Sindrup, S.; Staehelin Jensen, T. Management of Painful Neuropathies. Neurocutaneous Syndromes 2013, 115, 279–290. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Zeilhofer, H.U.; Acuña, M.A.; Gingras, J.; Yévenes, G.E. Glycine Receptors and Glycine Transporters: Targets for Novel Analgesics? Cell. Mol. Life Sci. 2018, 75, 447–465. [Google Scholar] [CrossRef]
- Eulenburg, V.; Armsen, W.; Betz, H.; Gomeza, J. Glycine Transporters: Essential Regulators of Neurotransmission. Trends Biochem. Sci. 2005, 30, 325–333. [Google Scholar] [CrossRef]
- Zafra, F.; Aragón, C.; Olivares, L.; Danbolt, N.C.; Giménez, C.; Storm-Mathisen, J. Glycine Transporters Are Differentially Expressed among CNS Cells. J. Neurosci. 1995, 15, 3952–3969. [Google Scholar] [CrossRef]
- Cubelos, B.; Giménez, C.; Zafra, F. Localization of the GLYT1 Glycine Transporter at Glutamatergic Synapses in the Rat Brain. Cereb. Cortex 2005, 15, 448–459. [Google Scholar] [CrossRef]
- Zafra, F.; Ibáñez, I.; Bartolomé-Martín, D.; Piniella, D.; Arribas-Blázquez, M.; Giménez, C. Glycine Transporters and Its Coupling with NMDA Receptors. Neurodegener. Dis. 2017, 16, 55–83. [Google Scholar] [CrossRef]
- Gomeza, J.; Ohno, K.; Hülsmann, S.; Armsen, W.; Eulenburg, V.; Richter, D.W.; Laube, B.; Betz, H. Deletion of the Mouse Glycine Transporter 2 Results in a Hyperekplexia Phenotype and Postnatal Lethality. Neuron 2003, 40, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Morita, K.; Motoyama, N.; Kitayama, T.; Morioka, N.; Kifune, K.; Dohi, T. Spinal Antiallodynia Action of Glycine Transporter Inhibitors in Neuropathic Pain Models in Mice. J. Pharmacol. Exp. Ther. 2008, 326, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armbruster, A.; Neumann, E.; Kötter, V.; Hermanns, H.; Werdehausen, R.; Eulenburg, V. The GlyT1 Inhibitor Bitopertin Ameliorates Allodynia and Hyperalgesia in Animal Models of Neuropathic and Inflammatory Pain. Front. Mol. Neurosci. 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Wiles, A.L.; Pearlman, R.-J.; Rosvall, M.; Aubrey, K.R.; Vandenberg, R.J. N-Arachidonyl-Glycine Inhibits the Glycine Transporter, GLYT2a. J. Neurochem. 2006, 99, 781–786. [Google Scholar] [CrossRef]
- Werdehausen, R.; Mittnacht, S.; Bee, L.A.; Minett, M.S.; Armbruster, A.; Bauer, I.; Wood, J.N.; Hermanns, H.; Eulenburg, V. The Lidocaine Metabolite N-Ethylglycine Has Antinociceptive Effects in Experimental Inflammatory and Neuropathic Pain. Pain 2015, 156, 1647–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centeno, M.V.; Mutso, A.; Millecamps, M.; Apkarian, V.A. Prefrontal Cortex and Spinal Cord Mediated Anti-Neuropathy and Analgesia Induced by Sarcosine, a Glycine-T1 Transporter Inhibitor. Pain 2009, 145, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Mingorance-Le Meur, A.; Ghisdal, P.; Mullier, B.; De Ron, P.; Downey, P.; Van Der Perren, C.; Declercq, V.; Cornelis, S.; Famelart, M.; Van Asperen, J.; et al. Reversible Inhibition of the Glycine Transporter GlyT2 Circumvents Acute Toxicity While Preserving Efficacy in the Treatment of Pain. Br. J. Pharmacol. 2013, 170, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Succar, R.; Mitchell, V.A.; Vaughan, C.L. Actions of N-Arachidonyl-Glycine in a Rat Inflammatory Pain Model. Mol. Pain 2007, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, R.J.; Ryan, R.M.; Carland, J.E.; Imlach, W.L.; Christie, M.J. Glycine Transport Inhibitors for the Treatment of Pain. Trends Pharmacol. Sci. 2014, 35, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Benke, D.; Yevenes, G.E. Chronic Pain States: Pharmacological Strategies to Restore Diminished Inhibitory Spinal Pain Control. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 111–133. [Google Scholar] [CrossRef]
- Acuña, M.A.; Yévenes, G.E.; Ralvenius, W.T.; Benke, D.; Lio, A.; Lara, C.O.; Muñoz, B.; Burgos, C.F.; Gustavo, M.-C.; Corringer, P.-J.; et al. Phosphorylation State–Dependent Modulation of Spinal Glycine Receptors Alleviates Inflammatory Pain. J. Clin. Investig. 2016, 126, 2547–2560. [Google Scholar] [CrossRef] [Green Version]
- Song, I.; Cho, S.; Nedeljkovic, S.S.; Lee, S.R.; Lee, C.; Kim, J.; Bai, S.J. Role of VVZ-149, a Novel Analgesic Molecule, in the Affective Component of Pain: Results from an Exploratory Proof-of-Concept Study of Postoperative Pain Following Laparoscopic and Robotic-Laparoscopic Gastrectomy. Pain Med. 2021. [Google Scholar] [CrossRef]
- McNicol, E.D.; Tzortzopoulou, A.; Cepeda, M.S.; Francia, M.B.D.; Farhat, T.; Schumann, R. Single-Dose Intravenous Paracetamol or Propacetamol for Prevention or Treatment of Postoperative Pain: A Systematic Review and Meta-Analysis. Br. J. Anaesth. 2011, 106, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Bannwarth, B.; Netter, P.; Lapicque, F.; Gillet, P.; Péré, P.; Boccard, E.; Royer, R.J.; Gaucher, A. Plasma and Cerebrospinal Fluid Concentrations of Paracetamol after a Single Intravenous Dose of Propacetamol. Br. J. Clin. Pharmacol. 1992, 34, 79–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guastella, J.; Brecha, N.; Weigmann, C.; Lester, H.; Davidson, N. Cloning, Expression, and Localization of a Rat Brain High-Affinity Glycine Transporter. Proc. Natl. Acad. Sci. USA 1992, 89, 7189–7193. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.R.; López-Corcuera, B.; Mandiyan, S.; Nelson, H.; Nelson, N. Cloning and Expression of a Spinal Cord- and Brain-Specific Glycine Transporter with Novel Structural Features. J. Biol. Chem. 1993, 268, 22802–22808. [Google Scholar] [CrossRef]
- Werdehausen, R.; Kremer, D.; Brandenburger, T.; Schlösser, L.; Jadasz, J.; Küry, P.; Bauer, I.; Aragón, C.; Eulenburg, V.; Hermanns, H. Lidocaine Metabolites Inhibit Glycine Transporter 1. J. Am. Soc. Anesthesiol. 2012, 116, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, R.J.; Shaddick, K.; Ju, P. Molecular Basis for Substrate Discrimination by Glycine Transporters. J. Biol. Chem. 2007, 282, 14447–14453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, A.; Singh, S.K.; Kawate, T.; Jin, Y.; Gouaux, E. Crystal Structure of a Bacterial Homologue of Na+/Cl--Dependent Neurotransmitter Transporters. Nat. Cell Biol. 2005, 437, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray Structure of Dopamine Transporter Elucidates Antidepressant Mechanism. Nat. Cell Biol. 2013, 503, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and Psychostimulant Recognition by the Dopamine Transporter. Nat. Cell Biol. 2015, 521, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray Structures and Mechanism of the Human Serotonin Transporter. Nat. Cell Biol. 2016, 532, 334–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.X.; Lyons-Warren, A.; Thio, L.L. The Glycine Transport Inhibitor Sarcosine Is an Inhibitory Glycine Receptor Agonist. Neuropharmacology 2009, 57, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Grudzinska, J.; Schemm, R.; Haeger, S.; Nicke, A.; Schmalzing, G.; Betz, H.; Laube, B. The β Subunit Determines the Ligand Binding Properties of Synaptic Glycine Receptors. Neuron 2005, 45, 727–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheipouri, D.; Gallagher, C.I.; Shimmon, S.; Rawling, T.; Vandenberg, R.J. A System for Assessing Dual Action Modulators of Glycine Transporters and Glycine Receptors. Biomolecules 2020, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Hyrc, K.; Thio, L.L. The Glycine Transport Inhibitor Sarcosine Is an NMDA Receptor Co-Agonist That Differs from Glycine. J. Physiol. 2009, 587, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.T.; Alexander, N.M.; Kelner, M.J. Definitive Liquid-Chromatographic Demonstration That N-Ethylglycine Is the Metabolite of Lidocaine That Interferes in the Kodak Sarcosine Oxidase-Coupled Method for Creatinine. Clin. Chem. 1988, 34, 2569–2572. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, P.H.; Fritsche, R.; Schliessbach, J.; Schmitt, B.; Arendt-Nielsen, L.; Zeilhofer, H.U.; Curatolo, M. Mutations Affecting Glycinergic Neurotransmission in Hyperekplexia Increase Pain Sensitivity. Brain 2018, 141, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.J.; Depner, U.B.; Wässle, H.; Ahmadi, S.; Heindl, C.; Reinold, H.; Smart, T.G.; Schütz, B.; Abo-Salem, O.M.; Zimmer, A.; et al. GlyR Alpha3: An Essential Target for Spinal PGE2-Mediated Inflammatory Pain Sensitization. Science 2004, 304, 884–887. [Google Scholar] [CrossRef] [Green Version]






| Construct | Plasmid | Restriction Enzyme | Concentration cRNA | Injection Volume | Incubation Time (d) |
|---|---|---|---|---|---|
| GlyT1 | pNKS2(CG1) pNKS2(NG1) | XbaI | 1 µg/µL | 46 nL | 4 |
| GlyT2 | pNKS2(CG2) pNKS2(NG2) | XbaI | 1 µg/µL | 46 nL | 4 |
| GlyRα1 | pNKS2(GlyRα1) | XbaI | 0.1 µg/µL | 46 nL | 1–2 |
| GlyRα2 | pNKS2(GlyRα2) | HindIII | 0.1 µg/µL | 46 nL | 1–2 |
| GlyRα3 | pNKS2(GlyRα3) | XbaI | 0.1 µg/µL | 46 nL | 1–2 |
| Construct | Glycine Dose–Response Relationship | Maximal Glycine-Induced Current Amplitude | |||
|---|---|---|---|---|---|
| EC50 (µM) | 95%CI (µM) | n | Mean ± SEM (nA) | n | |
| GlyT1 | 24.9 | 23.7–26.0 | 10–22 | 109 ± 4.2 | 148 |
| GlyT2 | 13.2 | 11.0–15.5 | 13–23 | 128 ± 6.3 | 130 |
| GlyRα1 | 162 | 141–186 | 12 | 6075 ± 971 | 47 |
| GlyRα2 | 325 | 289–362 | 3–15 | 3466 ± 387 | 45 |
| GlyRα3 | 234 | 220–248 | 11 | 2359 ± 106 | 40 |
| Construct | DEG Dose–Response Relationship (Variable Slope, Best Fit Values, Robust Fit) | |||
|---|---|---|---|---|
| EC50 | Lower 95%CI | Mean (IDEG/Imaxgly) ± SEM | n | |
| GlyT1 | >7.6 mM | 7.0 mM | 57.6 ± 2.5% | 8–26 |
| GlyT2 | >5.2 mM | 4.6 mM | 67.7 ± 4.4% | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barsch, L.; Werdehausen, R.; Leffler, A.; Eulenburg, V. Modulation of Glycinergic Neurotransmission may Contribute to the Analgesic Effects of Propacetamol. Biomolecules 2021, 11, 493. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11040493
Barsch L, Werdehausen R, Leffler A, Eulenburg V. Modulation of Glycinergic Neurotransmission may Contribute to the Analgesic Effects of Propacetamol. Biomolecules. 2021; 11(4):493. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11040493
Chicago/Turabian StyleBarsch, Lukas, Robert Werdehausen, Andreas Leffler, and Volker Eulenburg. 2021. "Modulation of Glycinergic Neurotransmission may Contribute to the Analgesic Effects of Propacetamol" Biomolecules 11, no. 4: 493. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11040493