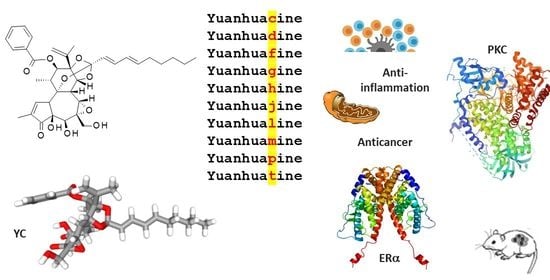
Yuanhuacin and Related Anti-Inflammatory and Anticancer Daphnane Diterpenes from Genkwa Flos—An Overview
Abstract
:1. Introduction
2. Yuanhuacin, a Potent Anticancer Agent
3. YC and Analogues. The yuanhuaXin Family (YX)
4. The Flavonoid Yuanhuanin (YN)
5. Conclusions
Funding
Conflicts of Interest
References
- Zhou, D.-C.; Zheng, G.; Jia, L.-Y.; He, X.; Zhang, C.-F.; Wang, C.-Z.; Yuan, C.-S. Comprehensive evaluation on anti-inflammatory and anti-angiogenic activities in vitro of fourteen flavonoids from Daphne genkwa based on the combination of efficacy coefficient method and principal component analysis. J. Ethnopharmacol. 2021, 268, 113683. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 3rd ed.; China Medical Science Press: Beijing, China, 2015; p. 159. [Google Scholar]
- Yu, J.-G.; Guo, J.; Zhu, K.Y.; Tao, W.; Chen, Y.; Liu, P.; Hua, Y.; Tang, Y.; Duan, J.-A. How impaired efficacy happened between Gancao and Yuanhua: Compounds, targets and pathways. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.-W.; Bao, Y.; Yu, H.; Chen, Q.-J.; Lu, F.; Zhai, S.; Zhang, C.-F.; Li, F.; Wang, C.-Z.; Yuan, C.-S. Anti-rheumatoid arthritis effects of flavonoids from Daphne genkwa. Int. Immunopharmacol. 2020, 83, 106384. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chou, G.; Hseu, Y.; Yang, H.; Kwan, H.; Yu, Z. Isolation of anticancer constituents from flos genkwa (Daphne genkwa Sieb.et Zucc.) through bioassay-guided procedures. Chem. Central J. 2013, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Sun, Q.; Hong, L.; Li, L.; Wu, Y.; Xia, M.; Ikejima, T.; Peng, Y.; Song, S. Daphnane-type diterpenes with inhibitory activities against human cancer cell lines from Daphne genkwa. Bioorganic Med. Chem. Lett. 2013, 23, 2500–2504. [Google Scholar] [CrossRef]
- Kwon, O.J.; Oh, H.C.; Lee, Y.J.; Kim, H.Y.; Tan, R.; Kang, D.G.; Lee, H.S. Sibjotang Increases Atrial Natriuretic Peptide Secretion in Beating Rabbit Atria. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, Z.-L.; Xu, J.-D.; Xu, Q.-Q.; Zhang, Y.; Guo, S.-J.; Yao, W.-F.; Bao, B.-H.; Tang, Y.-P.; Zhang, L. Toxicity reduction and water expelling effect preservation of Shizaotang after its toxic members processing with vinegar on rats with malignant pleural effusions. J. Ethnopharmacol. 2021, 268, 113583. [Google Scholar] [CrossRef]
- Jiang, Y.; Gu, L.; Zhang, R.; Zhang, Y.; Zhang, L.; Ju, P.; Ma, B.; Zhang, K.; Bi, K.; Chen, X. Evaluation of the indicative roles of seven potential biomarkers on hepato-nephrotoxicity induced by Genkwa Flos. J. Ethnopharmacol. 2014, 158, 317–324. [Google Scholar] [CrossRef]
- Li, L.; Yin, F.-Z.; Lu, T.-L.; Guan, H.-Y.; Cai, B.-C. Fingerprint of Vinegar Processed Genkwa Flos Based on Improving Euclidean Distance. Zhong yao cai 2015, 38, 1168–1171. [Google Scholar]
- Yun, J.W.; Kim, S.H.; Kim, Y.S.; You, J.R.; Kwon, E.; Jang, J.J.; Park, I.A.; Kim, H.C.; Kim, H.H.; Che, J.H.; et al. Evaluation of subchronic (13week) toxicity and genotoxicity potential of vinegar-processed Genkwa Flos. Regul. Toxicol. Pharmacol. 2015, 72, 386–393. [Google Scholar] [CrossRef]
- Geng, L.; Ma, C.; Zhang, L.; Yang, G.; Cui, Y.; Su, D.; Zhao, X.; Liu, Z.; Bi, K.; Chen, X. Metabonomic Study of Genkwa Flos-induced Hepatotoxicity and Effect of Herb-Processing Procedure on Toxicity. Phytother. Res. 2012, 27, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Sun, H.; Yuan, Y.; Liu, Z.; Cui, Y.; Bi, K.; Chen, X. Discrimination of raw and vinegar-processed Genkwa Flos using metabolomics coupled with multivariate data analysis: A discrimination study with metabolomics coupled with PCA. Fitoterapia 2013, 84, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Su, D.; Li, W.; Cai, B. Pharmacokinetic comparisons of six components from raw and vinegar-processed Daphne genkwa aqueous extracts following oral administration in rats by employing UHPLC–MS/MS approaches. J. Chromatogr. B 2018, 1079, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Ren, Q.; Tang, Y.-X.; Zhao, F.; Lin, B.; Huang, X.-X.; Song, S.-J. Sesquiterpenoids from the roots of Daphne genkwa Siebold et Zucc. With potential anti-inflammatory activity. Phytochemistry 2020, 174, 112348. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, W.-Y.; Shi, S.-C.; Han, F.-Y.; Zhang, Y.-Y.; Liu, Q.-B.; Yao, G.-D.; Lin, B.; Huang, X.-X.; Song, S.-J. Guaiane-Type Sesquiterpenoids from the Roots of Daphne genkwa and Evaluation of Their Neuroprotective Effects. J. Nat. Prod. 2019, 82, 1510–1517. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Q.; Zhang, Y.-Y.; Guo, R.; Lin, B.; Huang, X.-X.; Song, S.-J. Assignment of the stereostructures of sesquiterpenoids from the roots of Daphne genkwa via quantum chemical calculations. Fitoterapia 2019, 138, 104352. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.-B.; Hou, Z.-L.; Shi, S.-C.; Ren, H.; Yao, G.-D.; Lin, B.; Huang, X.-X.; Song, S.-J. Discovery of guaiane-type sesquiterpenoids from the roots of Daphne genkwa with neuroprotective effects. Bioorganic Chem. 2020, 95, 103545. [Google Scholar] [CrossRef]
- Jin, Y.-X.; Shi, L.-L.; Zhang, D.-P.; Wei, H.-Y.; Si, Y.; Ma, G.-X.; Zhang, J. A Review on Daphnane-Type Diterpenoids and Their Bioactive Studies. Molecules 2019, 24, 1842. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.-W.; Li, Y.; Luo, L.; Zhang, C.-Y.; Fan, W.; Gu, W.-Y.; Shi, K.-R.; Zhai, X.-X.; Zhu, J.-Y. Phytochemistry and Pharmacological Activities of the Diterpenoids from the Genus Daphne. Molecules 2021, 26, 6598. [Google Scholar] [CrossRef]
- Han, B.S.; Minh, N.V.; Choi, H.Y.; Byun, J.S.; Kim, W.G. Daphnane and Phorbol Diterpenes, Anti-neuroinflammatory Compounds with Nurr1 Activation from the Roots and Stems of Daphne genkwa. Biol. Pharm. Bull. 2017, 40, 2205–2211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-Y.; Shang, X.-Y.; Hou, X.-W.; Li, L.-Z.; Wang, W.; Hayashi, T.; Zhang, Y.; Yao, G.-D.; Song, S.-J. Yuanhuatine from Daphne genkwa selectively induces mitochondrial apoptosis in estrogen receptor α-positive breast cancer cells in vitro. Planta Med. 2019, 85, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.P.; Wang, C.S.; Chou, P.N.; Pan, P.C.; Liu, J.S. Studies on the active principles in the root of Yuan-Hua (Daphne gemkwa). I. Isolation and structure of yuanhuacine. Acta Chim. Sin. (Hua Hsueh Hsueh Pao) 1977, 35, 103–108, (Chem. Abs. 1978, 89, 39369). [Google Scholar]
- Zhou, B.-N.; Xue, L.; Lin, L.-Z.; Lin, L.-J.; Johnson, M.E.; Cordell, G.A. NMR assignments and conformational analysis of yuanhuacin. Magn. Reson. Chem. 1993, 31, 194–199. [Google Scholar] [CrossRef]
- Yang, B.Y. Mechanism of the action of Yuanhuacine to induce labor during mid pregnancy (author’s transl). Zhonghua yi xue za zhi 1981, 61, 613–616. [Google Scholar]
- Ding, G.S. Important Chinese herbal remedies. Clin. Ther. 1987, 9, 345–357. [Google Scholar]
- Liang, Y.G. Morphological observations of placenta in 56 cases of mid-term abortion induced by Yuanhua preparations (author’s transl). Zhonghua fu chan ke za zhi 1979, 14, 290–292. [Google Scholar]
- Huang, B. Comparison of 4 methods of midterm termination and their maternal influence (author’s transl). Tianjin Yi Yao 1982, 10, 284–287. [Google Scholar]
- Zhang, J.G. The clinical and pathological study of induced abortion by Yuanhuacine film in 12–16 week pregnancy. Sheng zhi yu bi yun 1987, 7, 65–66. [Google Scholar]
- Li, P.Q. Yuanhuacin film for menstruation induction and termination of early pregnancy: Analysis of 382. Zhonghua fu chan ke za zhi 1989, 24, 231–233. [Google Scholar]
- Yan, R.; Li, P.Q. Preparation of yuanhuacine by low pressure column chromatography. China J. Chin. Mater. Medica 1993, 18, 729. [Google Scholar]
- Zhang, B.X.; Yuan, X.T.; Xia, K.; Li, J.H. Determination of yuanhuacine in Daphne genkwa Sieb. et Zucc. by HPLC. China J. Chin. Mater. Medica 1994, 19, 551–552. [Google Scholar]
- Hallx, I.; Kasai, R.; Wu, R.; Tagahara, K.; Lee, K. Antitumor Agents LV: Effects of Genkwadaphnin and Yuanhuacine on Nucleic Acid Synthesis of P-388 Lymphocytic Leukemia Cells. J. Pharm. Sci. 1982, 71, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.F.; Hall, I.H.; Lee, K.H. Antitumor Agents LVI: The Protein Synthesis Inhibition by Genkwadaphnin and Yuanhuacine of P-388 Lymphocytic Leukemia Cells. J. Pharm. Sci. 1982, 71, 1340–1344. [Google Scholar] [CrossRef]
- Zhang, D.C.; Cao, C.X.; Zhang, C.J.; Zhou, B.N. Yuanhuacin A is a selective antagonist of phorbol ester receptor in protein kinase C. Sci. China Ser. B Chem. Life Sci. Earth Sci. 1993, 36, 803–808. [Google Scholar]
- Zhang, S.; Li, X.; Zhang, F.; Yang, P.; Gao, X.; Song, Q. Preparation of yuanhuacine and relative daphne diterpene esters from Daphne genkwa and structure–activity relationship of potent inhibitory activity against DNA topoisomerase I. Bioorganic Med. Chem. 2006, 14, 3888–3895. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Liou, Y.-F.; Oswald, C.; Lee, K.-H. The effects of Genkwadaphnin and Gnidilatidin on the growth of P-388, L-1210 leukemia and KB carcinoma cells in Vitro. Eur. J. Cancer Clin. Oncol. 1986, 22, 45–52. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Nam, J.-W.; Seo, E.-K.; Lee, S.K. Daphnane Diterpene Esters with Anti-proliferative Activities against Human Lung Cancer Cells from Daphne genkwa. Chem. Pharm. Bull. 2010, 58, 234–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.I.; Hong, J.-Y.; Lee, H.-J.; Bae, S.Y.; Jung, C.; Park, H.J.; Lee, S.K. Anti-Tumor Activity of Yuanhuacine by Regulating AMPK/mTOR Signaling Pathway and Actin Cytoskeleton Organization in Non-Small Cell Lung Cancer Cells. PLoS ONE 2015, 10, e0144368. [Google Scholar] [CrossRef] [Green Version]
- Park, B.-Y.; Min, B.-S.; Ahn, K.-S.; Kwon, O.-K.; Joung, H.; Bae, K.-H.; Lee, H.-K.; Oh, S.-R. Daphnane diterpene esters isolated from flower buds of Daphne genkwa induce apoptosis in human myelocytic HL-60 cells and suppress tumor growth in Lewis lung carcinoma (LLC)-inoculated mouse model. J. Ethnopharmacol. 2007, 111, 496–503. [Google Scholar] [CrossRef]
- Bang, K.K.; Yun, C.-Y.; Lee, C.; Jin, Q.; Lee, J.W.; Jung, S.-H.; Lee, D.; Lee, M.K.; Hong, J.T.; Kim, Y.; et al. Melanogenesis inhibitory daphnane diterpenoids from the flower buds of Daphne genkwa. Bioorganic Med. Chem. Lett. 2013, 23, 3334–3337. [Google Scholar] [CrossRef] [PubMed]
- Fermaintt, C.S.; Peramuna, T.; Cai, S.; Takahashi-Ruiz, L.; Essif, J.N.; Grant, C.V.; O’Keefe, B.R.; Mooberry, S.L.; Cichewicz, R.H.; Risinger, A.L. Yuanhuacine Is a Potent and Selective Inhibitor of the Basal-Like 2 Subtype of Triple Negative Breast Cancer with Immunogenic Potential. Cancers 2021, 13, 2834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Li, J.; Jin, H.; Song, S.; Huang, C. The Chinese herb isolate yuanhuacine (YHL-14) induces G2/M arrest in human cancer cells by up-regulating p21 protein expression through an p53 protein-independent cascade. J. Biol. Chem. 2014, 289, 6394–6403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, L.; Fresco, P.; Pinto, E.; Portugal, H.; Gonçalves, J. Differential activation by daphnetoxin and mezerein of PKC-isotypes alpha, beta I., delta and zeta. Planta Med. 2001, 67, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, S.; Wada, K.; Munakata, K. Odoracin, a nematicidal constituent from Daphne odora. Agric. Biol. Chem. 1976, 40, 2119–2120. [Google Scholar] [CrossRef]
- Ohigashi, H.; Hirota, M.; Sugimura, T.; Ohtsuka, T.; Koshimizu, K.; Fujiki, H.; Suganuma, M.; Yamaizumi, Z.; Sugimura, T. Resiniferonol-related Diterpene Esters from Daphne odora Thunb. and their Ornithine Decarboxylase-inducing Activity in Mouse Skin. Agricult. Biol. Chem. 1982, 46, 2605–2608. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Shizuri, Y.; Summer, W.C.; Haynes Jr, H.R.; Leighton, A.P.; Sickles, B.R. Isolation and structural elucidation of new potent antileukemic diterpenoid esters from Gnidia species. J. Org. Chem. 1976, 41, 3850–3853. [Google Scholar] [CrossRef]
- Fujita, E.; Fuji, K.; Nagao, Y.; Node, M.; Ochiai, M. The Chemistry on Diterpenoids in 1976. Bull. Inst. Chem. Res. 1977, 55, 494–538. [Google Scholar]
- Borris, R.P.; Cordell, G.A. Studies of the Thymelaeaceae II. Antineoplastic Principles of Gnidia kraussiana. J. Nat. Prod. 1984, 47, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, N.; Cordell, G.; Kaas, C. What is odoratin? J. Pharm. Sci. 1980, 69, 1107. [Google Scholar] [CrossRef]
- Thurlow, K.J. Chemical Nomenclature; Springer: Dordrecht, The Netherlands, 1998; p. 168. [Google Scholar]
- Shigemori, H.; Nakasone, R.; Kurisu, M.; Onodera, M.; Miyamae, Y.; Matsuura, D.; Kanatani, H.; Yano, S. Promoting Effects on Hepatocyte Growth Factor Production of Daphnane Diterpenoids from Daphne odora. HETEROCYCLES 2013, 87, 1087. [Google Scholar] [CrossRef]
- Matsuyama, K.; Miyamae, Y.; Sekii, Y.; Han, J.; Abderrabba, M.; Morio, T.; Shigemori, H.; Isoda, H. Effect of Mediterranean Medicinal Plant Extracts on Melanogenesis Regulation. J. Arid. Land Studies 2009, 19, 387–390. [Google Scholar]
- Villareal, M.O.; Sato, Y.; Matsuyama, K.; Isoda, H. Daphnane diterpenes inhibit the metastatic potential of B16F10 murine melanoma cells in vitro and in vivo. BMC Cancer. 2018, 18, 856. [Google Scholar]
- Qiao, Y.; Zhao, Y.; Wu, Q.; Sun, L.; Ruan, Q.; Chen, Y.; Wang, M.; Duan, J.; Wang, D. Full Toxicity Assessment of Genkwa Flos and the Underlying Mechanism in Nematode Caenorhabditis elegans. PLoS ONE 2014, 9, e91825. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Guo, J.-M.; Qian, Y.-F.; Guo, S.; Ma, C.-H.; Duan, J.-A. Toxicity of daphnane-type diterpenoids from Genkwa Flos and their pharmacokinetic profile in rat. Phytomedicine 2013, 21, 82–89. [Google Scholar] [CrossRef]
- Li, M.; Duan, J.; Hu, R.; Chen, Z. Preparation of dry powder inhalation of yuanhuacine and its tissue distribution in rats. J. China Pharm. Univ. 2017, 6, 297–304. [Google Scholar]
- Li, M.; Liu, X.; Cai, H.; Shen, Z.; Xu, L.; Li, W.; Wu, L.; Duan, J.; Chen, Z. Validation and Application of an Ultra High-Performance Liquid Chromatography Tandem Mass Spectrometry Method for Yuanhuacine Determination in Rat Plasma after Pulmonary Administration: Pharmacokinetic Evaluation of a New Drug Delivery System. Molecules 2016, 21, 1733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gao, X.; Shen, K.; Yang, P.; Ju, X. Evaluation of poly(d,l-lactide-co-glycolide) microspheres for the lung-targeting of yuanhuacine, a novel DNA topoisomerase I inhibitor. J. Drug Target. 2009, 17, 286–293. [Google Scholar] [CrossRef]
- Chen, R.; Xu, L.; Fan, Q.; Li, M.; Wang, J.; Wu, L.; Li, W.; Duan, J.; Chen, Z. Hierarchical pulmonary target nanoparticles via inhaled administration for anticancer drug delivery. Drug Deliv. 2017, 24, 1191–1203. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.T.; Chen, C.H.; Yin, P.P.; Pan, P.C. Studies on the active principles in the root of Yuan-Hua (Daphne genkwa). II. Isolation and structure of a new antifertile diterpene orthoester, yuanhuadine. Chin. Pharm. Bull. 1980, 15, 39. [Google Scholar]
- Wang, C.R.; Chen, Z.X.; Ying, B.P.; Zhou, B.N.; Liu, J.S.; Pan, B.C. Studies on the active principles in the root of Yuan-Hua (Daphne genkwa). II. Isolation and structure of a new antifertile diterpene yuanhuadine. Acta Chim. Sin. 1981, 39, 421–426. [Google Scholar]
- Wang, C.R.; Huang, H.Z.; Xu, R.S.; Dou, Y.Y.; Wu, X.C.; Li, Y. Isolation and structure of a new diterpene orthester, yuanhuafine. Chin. Pharm. Bull. 1982, 17, 174. [Google Scholar]
- Wang, C.R.; Huang, H.Z.; Xu, R.S.; Dou, Y.Y.; Wu, X.C.; Li, Y. Studies on the active principles in the root of Yuan-Hua (Daphne gemkwa). III. Isolation and structure of yuanhuafine. Acta Chim. Sin. 1982, 40, 835–839. [Google Scholar]
- Hu, B.H.; Sha, H.; Wang, C.R.; Yu, D.F.; Wu, X.C.; Yu, X.G. Antifertility constituent of the flower Yuan-Hua. Isolation and structure of yuanhuatine. Acta Chim. Sin. 1985, 43, 460–462. [Google Scholar]
- Sha, H.; He, Z.W.; Wu, X.C. Constituents of the Yuanhua’s flower buds—isolation and structure of yuanhuapine. Acta Chim. Sin. 1986, 44, 843–845. [Google Scholar]
- Wang, Q.W. Clinico-pathologic study on the action of yuanhuadine in mid-trimester abortion. Zhonghua fu chan ke za zhi 1983, 18, 154–156. [Google Scholar]
- Zhang, S.; Zhang, F.; Li, X.; Dong, W.; Wen, L.; Wang, S. Evaluation of Daphne genkwa diterpenes: Fingerprint and quantitative analysis by high performance liquid chromatography. Phytochem. Anal. 2006, 18, 91–97. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Chung, H.-J.; Lee, H.-J.; Park, H.J.; Lee, S.K. Growth Inhibition of Human Lung Cancer Cells via Down-regulation of Epidermal Growth Factor Receptor Signaling by Yuanhuadine, a Daphnane Diterpene from Daphne genkwa. J. Nat. Prod. 2011, 74, 2102–2108. [Google Scholar] [CrossRef]
- Kim, D.; Bach, D.-H.; Fan, Y.-H.; Luu, T.-T.-T.; Hong, J.-Y.; Park, H.J.; Lee, S.K. AXL degradation in combination with EGFR-TKI can delay and overcome acquired resistance in human non-small cell lung cancer cells. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jeong, I.; Song, J.; Bae, S.Y.; Lee, S.K. Overcoming the Intrinsic Gefitinib-resistance via Downregulation of AXL in Non-small Cell Lung Cancer. J. Cancer Prev. 2019, 24, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.Y.; Hong, J.-Y.; Lee, H.-J.; Park, H.J.; Lee, S.K. Targeting the degradation of AXL receptor tyrosine kinase to overcome resistance in gefitinib-resistant non-small cell lung cancer. Oncotarget 2015, 6, 10146–10160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.Y.; Park, H.J.; Hong, J.-Y.; Lee, H.-J.; Lee, S.K. Down-regulation of SerpinB2 is associated with gefitinib resistance in non-small cell lung cancer and enhances invadopodia-like structure protrusions. Sci. Rep. 2016, 6, 32258. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Kim, D.; Bae, S.Y.; Kim, W.K.; Hong, J.-Y.; Lee, H.-J.; Rajasekaran, N.; Kwon, S.; Fan, Y.; Luu, T.-T.-T.; et al. Targeting Nicotinamide N-Methyltransferase and miR-449a in EGFR-TKI-Resistant Non-Small-Cell Lung Cancer Cells. Mol. Ther.—Nucleic Acids 2018, 11, 455–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, D.-H.; Luu, T.-T.-T.; Kim, D.; An, Y.J.; Park, S.; Park, H.J.; Lee, S.K. BMP4 Upregulation Is Associated with Acquired Drug Resistance and Fatty Acid Metabolism in EGFR-Mutant Non-Small-Cell Lung Cancer Cells. Mol. Ther.—Nucleic Acids 2018, 12, 817–828. [Google Scholar] [CrossRef] [Green Version]
- Luu, T.-T.-T.; Bach, D.-H.; Kim, D.; Hu, R.; Park, H.J.; Lee, S.K. Overexpression of AGR2 Is Associated with Drug Resistance in Mutant Non-small Cell Lung Cancers. Anticancer Res. 2020, 40, 1855–1866. [Google Scholar] [CrossRef]
- Wang, L.; Lan, X.-Y.; Ji, J.; Zhang, C.-F.; Li, F.; Wang, C.-Z.; Yuan, C.-S. Anti-inflammatory and anti-angiogenic activities in vitro of eight diterpenes from Daphne genkwa based on hierarchical cluster and principal component analysis. J. Nat. Med. 2018, 72, 675–685. [Google Scholar] [CrossRef]
- Li, L.-Z.; Gao, P.-Y.; Peng, Y.; Wang, L.-H.; Yang, J.-Y.; Wu, C.-F.; Zhang, Y.; Song, S.-J. Daphnane-Type Diterpenoids from the Flower Buds of Daphne genkwa. Helvetica Chim. Acta 2010, 93, 1172–1179. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, J.; Tang, Y.; Wu, L.; Tao, W.; Qian, Y.; Duan, J.-A. Pharmacokinetic profile and metabolite identification of yuanhuapine, a bioactive component in Daphne genkwa by ultra-high performance liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015, 112, 60–69. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, J.-A.; Guo, J.; Shang, E.; Tang, Y.; Qian, Y.; Tao, W.; Liu, P. Yuanhuapine-induced intestinal and hepatotoxicity were correlated with disturbance of amino acids, lipids, carbohydrate metabolism and gut microflora function: A rat urine metabonomic study. J. Chromatogr. B 2016, 1026, 183–192. [Google Scholar] [CrossRef]
- Wender, P.A.; Buschmann, N.; Cardin, N.B.; Jones, L.R.; Kan, C.; Kee, J.-M.; Kowalski, J.A.; Longcore, K.E. Gateway synthesis of daphnane congeners and their protein kinase C affinities and cell-growth activities. Nat. Chem. 2011, 3, 615–619. [Google Scholar] [CrossRef] [Green Version]
- Phillips, A.J. Bioactive natural products: Function first. Nat. Chem. 2011, 3, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Kuok, C.-F.; Hoi, S.-O.; Hoi, C.-F.; Chan, C.-H.; Fong, I.-H.; Ngok, C.-K.; Meng, L.-R.; Fong, P. Synergistic antibacterial effects of herbal extracts and antibiotics on methicillin-resistant Staphylococcus aureus: A computational and experimental study. Exp. Biol. Med. 2017, 242, 731–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, P.Y.; Chow, S.; Somerville, M.J.; Fletcher, M.T.; De Voss, J.J. Daphnane- and Tigliane-Type Diterpenoid Esters and Orthoesters from Pimelea elongata. J. Nat. Prod. 2010, 73, 1907–1913. [Google Scholar] [CrossRef]
- Jo, S.-K.; Hong, J.-Y.; Park, H.J.; Lee, S.K. Anticancer Activity of Novel Daphnane Diterpenoids from Daphne genkwa through Cell-Cycle Arrest and Suppression of Akt/STAT/Src Signalings in Human Lung Cancer Cells. Biomol. Ther. 2012, 20, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.-D.; Lu, Y.; Yan, M.; Chen, C.-H.; Morris-Natschke, S.L.; Lee, K.-H.; Chen, D.-F. Rapid Recognition and Targeted Isolation of Anti-HIV Daphnane Diterpenes from Daphne genkwa Guided by UPLC-MSn. J. Nat. Prod. 2019, 83, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-B.; Lee, H.-R.; Jee, D.J.; Shin, S.-H.; Nah, S.-S.; Yoon, S.Y.; Kim, J.W. PRDM1, a Tumor-Suppressor Gene, is Induced by Genkwadaphnin in Human Colon Cancer SW620 Cells. J. Cell. Biochem. 2016, 117, 172–179. [Google Scholar] [CrossRef]
- Wu, J.; Guo, L.; Qiu, X.; Ren, Y.; Li, F.; Cui, W.; Song, S. Genkwadaphnin inhibits growth and invasion in hepatocellular carcinoma by blocking DHCR24-mediated cholesterol biosynthesis and lipid rafts formation. Br. J. Cancer 2020, 123, 1673–1685. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Lee, S.K.; Bae, S.Y.; Kim, J.; Kim, M.; Kil, W.H.; Kim, S.W.; Lee, J.E.; Nam, S.J. Protein kinase C-α downregulates estrogen receptor-α by suppressing c-Jun phosphorylation in estrogen receptor-positive breast cancer cells. Oncol. Rep. 2013, 31, 1423–1428. [Google Scholar] [CrossRef]
- Yun, J.; Pannuti, A.; Espinoza, I.; Zhu, H.; Hicks, C.; Zhu, X.; Caskey, M.; Rizzo, P.; D’Souza, G.; Backus, K.; et al. Crosstalk between PKCalpha and Notch-4 in endocrine-resistant breast cancer cells. Oncogenesis 2013, 2, 60. [Google Scholar] [CrossRef]
- Li, S.F.; Wang, Z.X. Isolation and identification of Yuan Hua (Daphne genkwa) flavonoids. Chin. Tradit. Herb. Drugs 1983, 14, 392–394. [Google Scholar]
- Ji, C.; Liu, Y.; Feng, W.; Wang, M.; Zhao, T. Flavonoids in the Yuanhua Leaf (Daphne genkwa). Zhangcaoyao 1986, 17, 487–489, (Chem. Abs. 1987, 106, 116524k). [Google Scholar]
- Tosun, A. Türlerinin kimyasal içeriği ve biyolojik aktiviteleri: Chemical constituents and biological activities of Daphne L. Species. Ank. Univ. Eczaci. Fak. Derg. 1994, 35, 001–023. [Google Scholar] [CrossRef]
- Ulubelen, A.; Bucker, R.; Mabry, T.J. Flavone 5-O-glucosides from Daphne sericea. Phytochemistry 1982, 21, 801–803. [Google Scholar] [CrossRef]
- Vankar, P.S.; Shanker, R.; Dixit, S.; Mahanta, D.; Tiwari, S.C. Chemical characterization of extract derived from Daphne papyraceae and sonicator dyeing of cotton, silk and wool with the extract. Pigment Resin Technol. 2009, 38, 181–187. [Google Scholar] [CrossRef]
- Xu, W.; Jin, H.; Zhang, W.; Hu, X.; Zhang, W.; Fu, J.; Su, J.; Yan, S.; Shen, Y. Chemical constituents from Daphne pedunculata. Chem. Nat. Compd. 2009, 45, 417–419. [Google Scholar] [CrossRef]
- Xu, W.; Shen, J.-G.; Jiang, J.-Q. Phytochemical and Biological Studies of the Plants from the Genus Daphne. Chem. Biodivers. 2011, 8, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Venditti, A.; De Vita, D.; Sciubba, F.; Tomai, P.; Franceschin, M.; Di Cecco, M.; Ciaschetti, G.; Di Sotto, A.; Stringaro, A.; et al. Phytochemical Analysis and Biological Activities of the Ethanolic Extract of Daphne sericea Vahl Flowering Aerial Parts Collected in Central Italy. Biomolecules 2021, 11, 379. [Google Scholar] [CrossRef]
- Zheng, W.; Gao, X.; Chen, C.; Tan, R. Total flavonoids of Daphne genkwa root significantly inhibit the growth and metastasis of Lewis lung carcinoma in C57BL6 mice. Int. Immunopharmacol. 2007, 7, 117–127. [Google Scholar] [CrossRef]
- Devkota, H.P.; Watanabe, M.; Watanabe, T.; Yahara, S. Phenolic Compounds from the Aerial Parts of Diplomorpha canescens. Chem. Pharm. Bull. 2012, 60, 554–556. [Google Scholar] [CrossRef] [Green Version]
- Tewtrakul, S.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T.; Yoshinaga, T.; Fujiwara, T.; Supavita, T.; Yuenyongsawad, S.; et al. HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytotherapy Res. 2003, 17, 232–239. [Google Scholar] [CrossRef]
- Qawoogha, S.S.; Shahiwala, A. Identification of potential anticancer phytochemicals against colorectal cancer by structure-based docking studies. J. Recept. Signal Transduct. 2020, 40, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Tang, Y.-P.; Shang, E.-X.; Zhu, Z.-H.; Tao, W.-W.; Yu, J.-G.; Feng, L.-M.; Yang, J.; Wang, J.; Su, S.-L.; et al. Incompatibility assessment of Genkwa Flos and Glycyrrhizae Radix et Rhizoma with biochemical, histopathological and metabonomic approach. J. Ethnopharmacol. 2018, 229, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, D.; Liang, Y.; Zhang, Z.; Guo, J.; Chen, Y.; Yan, Y.; Liu, H.; Lei, L.; Wang, Z.; et al. Licorice-Yuanhua Herbal Pair Induces Ileum Injuries Through Weakening Epithelial and Mucous Barrier Functions: Saponins, Flavonoids, and Di-Terpenes All Involved. Front. Pharmacol. 2020, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Y.; Guo, J.; Tao, W.; Chen, Y.; Fan, X.; Shen, J.; Duan, J.-A. Health risk of Licorice-Yuanhua combination through induction of colonic H2S metabolism. J. Ethnopharmacol. 2019, 236, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.-L.; Yao, G.-D.; Song, S.-J. Daphnane-type diterpenes from genus Daphne and their anti-tumor activity. Chin. Herb. Med. 2021, 13, 145–156. [Google Scholar] [CrossRef]
- Pan, R.-R.; Zhang, C.-Y.; Li, Y.; Zhang, B.-B.; Zhao, L.; Ye, Y.; Song, Y.-N.; Zhang, M.; Tie, H.-Y.; Zhang, H.; et al. Daphnane Diterpenoids from Daphne genkwa Inhibit PI3K/Akt/mTOR Signaling and Induce Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells. J. Nat. Prod. 2020, 83, 1238–1248. [Google Scholar] [CrossRef]
- Hou, X.-W.; Han, S.; Zhang, Y.-Y.; Su, H.-B.; Gao, P.-Y.; Li, L.-Z.; Song, S.-J. Neogenkwanine I from the flower buds of Daphne genkwa with its stereostructure confirmation using quantum calculation profiles and antitumor evaluation. Nat. Prod. Res. 2018, 34, 405–412. [Google Scholar] [CrossRef]
- Van Minh, N.; Han, B.S.; Choi, H.Y.; Byun, J.; Park, J.S.; Kim, W.G. Genkwalathins A and B, new lathyrane-type diterpenes from Daphne genkwa. Nat. Prod. Res. 2018, 32, 1782–1790. [Google Scholar] [CrossRef]






| Cellular or Tumor Models | Observed Effects | References |
|---|---|---|
| Melanoma cells | YC and YD inhibit melanogenesis and B16 melanoma cell proliferation in vitro. | [42] |
| Breast cancer model (BC) | BC growth inhibition in vivo (MCF7 model) with a Genkwa Flos extract and characterization of YC | [6] |
| Triple-negative breast cancer cell (TNBC), basal-like 2 subtype | Potent activity of YC against the BL2 subtype of TNBC (nM IC50 against HCC1806 and HCC70 cell lines). | [43] |
| Human non-small cell lung cancer (NSCLC) | YC inhibits cell growth and actin cytoskeleton organization and reduces tumor growth in vivo (H1993 model). | [40] |
| Comparison of YC, YD, YG, YL as inhibitors of A549 cell proliferation. | [39] | |
| YC inhibits Lewis lung carcinoma (LLC) growth in mice. | [41] | |
| Colon cancer cells | Inhibition of HCT116 cell proliferation and G2/M cell cycle arrest induced by YC, via upregulation of p21 and downregulation of Sp1. | [44] |
| Promyelocytic leukemia | YC inhibits proliferation of P-388 and l-1210 murine leukemia cells in vitro. | [38] |
| YC inhibits HL-60 cell growth and induces apoptosis. | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C. Yuanhuacin and Related Anti-Inflammatory and Anticancer Daphnane Diterpenes from Genkwa Flos—An Overview. Biomolecules 2022, 12, 192. https://0-doi-org.brum.beds.ac.uk/10.3390/biom12020192
Bailly C. Yuanhuacin and Related Anti-Inflammatory and Anticancer Daphnane Diterpenes from Genkwa Flos—An Overview. Biomolecules. 2022; 12(2):192. https://0-doi-org.brum.beds.ac.uk/10.3390/biom12020192
Chicago/Turabian StyleBailly, Christian. 2022. "Yuanhuacin and Related Anti-Inflammatory and Anticancer Daphnane Diterpenes from Genkwa Flos—An Overview" Biomolecules 12, no. 2: 192. https://0-doi-org.brum.beds.ac.uk/10.3390/biom12020192