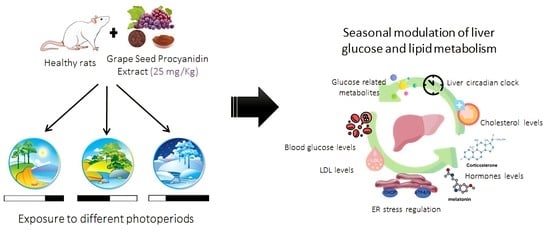
Grape-Seed Procyanidin Extract (GSPE) Seasonal-Dependent Modulation of Glucose and Lipid Metabolism in the Liver of Healthy F344 Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape Seed Proanthocyanidin-Rich Extract
2.2. Experimental Design
2.3. Serum Analysis
2.4. RNA Extraction
2.5. Gene Expression Analysis
2.6. Extraction and Measurement of Concentrations of Lipids in Liver
2.7. Protein Extraction
2.8. Determination of Serum Hormones
2.9. Metabolomic Analysis
2.10. Statistical Analysis
3. Results
3.1. Photoperiod Strongly Influences Body Weight Gain of F344 Rats
3.2. The Effect of GSPE on Serum Parameters Depends on Photoperiod
3.3. The Exposure to Different Photoperiods Altered the Expression of Circadian Rhythm-Related Genes in the Liver of GSPE-Treated Rats
3.4. Melatonin and Hormones from the Hypothalamus Pituitary Adrenal (HPA) Axes Have a Seasonal Variation which Is Affected by GSPE Consumption
3.5. Lipid Liver Profile
3.6. mRNA Levels of Key Hepatic Lipid and Glucose-Metabolic Regulators Varies due to Photoperiod and GSPE Treatment
3.7. Analysis of Glucose-Related Metabolites Reveals Photoperiod Influence over GSPE Effect
3.8. Photoperiod Affects the GSPE Effect on ER Stress Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, S.; Loudon, A.; Wood, S.; Loudon, A. Clocks for All Seasons: Unwinding the Roles and Mechanisms of Circadian and Interval Timers in the Hypothalamus and Pituitary. J. Endocrinol. 2014, 222, R39–R59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lincoln, G.A.; Anderson, H.; Loudon, A. Clock Genes in Calendar Cells as the Basis of Annual Timekeeping in Mammals—A Unifying Hypothesis. J. Endocrinol. 2003, 179, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendt, J. Melatonin, Circadian Rhythms, and Sleep. N. Engl. J. Med. 2000, 343, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Dopico, X.C.; Evangelou, M.; Ferreira, R.C.; Guo, H.; Pekalski, M.L.; Smyth, D.J.; Cooper, N.; Burren, O.S.; Fulford, A.J.; Hennig, B.J.; et al. Widespread Seasonal Gene Expression Reveals Annual Differences in Human Immunity and Physiology. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Takami, T.; Shintani, H.; Sasai, N.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Seasonal Variations in Photoperiod Affect Hepatic Metabolism of Medaka (Oryzias Latipes). FEBS Open Bio 2021, 11, 1029–1040. [Google Scholar] [CrossRef]
- Togo, Y.; Otsuka, T.; Goto, M.; Furuse, M.; Yasuo, S. Photoperiod Regulates Dietary Preferences and Energy Metabolism in Young Developing Fischer 344 Rats but Not in Same-Age Wistar Rats. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Chainy, G.B.N.; Paital, B.; Dandapat, J. An Overview of Seasonal Changes in Oxidative Stress and Antioxidant Defence Parameters in Some Invertebrate and Vertebrate Species. Scientifica 2016, 2016, 6126570. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Carrión, Á.; de Azua, M.J.R.; Mulero, M.; Arola-Arnal, A.; Suárez, M. Oxidative Stress in Rats Is Modulated by Seasonal Consumption of Sweet Cherries from Different Geographical Origins: Local vs. Non-Local. Nutrients 2020, 12, 2854. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Cos, P.; Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.; Vlietinck, A. Proanthocyanidins in Health Care: Current and New Trends. Curr. Med. Chem. 2012, 11, 1345–1359. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. What Is Xenohormesis? Am. J. Pharmacol. Toxicol. 2008, 3, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokozawa, T.; Lee1, Y.A.; Cho, E.J.; Matsumoto, K.; Park, C.H.; Shibahara, N. Anti-Aging Effects of Oligomeric Proanthocyanidins Isolated from Persimmon Fruits. Drug Discov. 2011, 5, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)Ever after: Aging in the Context of Oxidative Stress, Proteostasis Loss and Cellular Senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.C.; Grosso, R.A.; Fader, C.M. Hallmarks of Aging: An Autophagic Perspective. Front. Endocrinol. 2019, 10, 790. [Google Scholar] [CrossRef]
- Okudan, N.; Barışkaner, H.; Gökbel, H.; Şahin, A.S.; Belviranlı, M.; Baysal, H. The Effect of Supplementation of Grape Seed Proanthocyanidin Extract on Vascular Dysfunction in Experimental Diabetes. J. Med. Food 2011, 14, 1298–1302. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, X.; Jiang, Y.; Zhang, Z.; Bao, L.; Li, Y.; Zhang, F.; Ma, X.; Cai, X.; Jing, L.; et al. Grape Seed Proanthocyanidin Extracts Alleviate Oxidative Stress and ER Stress in Skeletal Muscle of Low-Dose Streptozotocin- and High-Carbohydrate/High-Fat Diet-Induced Diabetic Rats. Mol. Nutr. Food Res. 2013, 57, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Pajuelo, D.; Quesada, H.; Díaz, S.; Fernández-Iglesias, A.; Arola-Arnal, A.; Bladé, C.; Salvadó, J.; Arola, L. Chronic Dietary Supplementation of Proanthocyanidins Corrects the Mitochondrial Dysfunction of Brown Adipose Tissue Caused by Diet-Induced Obesity in Wistar Rats. Br. J. Nutr. 2012, 107, 170–178. [Google Scholar] [CrossRef]
- Fernández-Iglesias, A.; Pajuelo, D.; Quesada, H.; Díaz, S.; Bladé, C.; Arola, L.; Salvadó, M.J.; Mulero, M. Grape Seed Proanthocyanidin Extract Improves the Hepatic Glutathione Metabolism in Obese Zucker Rats. Mol. Nutr. Food Res. 2014, 58, 727–737. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Zhang, Z.; Dai, X.; Jiang, Y.; Bao, L.; Li, Y. Grape Seed Proanthocyanidins Ameliorate Pancreatic Beta-Cell Dysfunction and Death in Low-Dose Streptozotocin- and High-Carbohydrate/High-Fat Diet-Induced Diabetic Rats Partially by Regulating Endoplasmic Reticulum Stress. Nutr. Metab. 2013, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Ibars, M.; Ardid-Ruiz, A.; Suárez, M.; Muguerza, B.; Bladé, C.; Aragonès, G. Proanthocyanidins Potentiate Hypothalamic Leptin/STAT3 Signalling and Pomc Gene Expression in Rats with Diet-Induced Obesity. Int. J. Obes. 2016, 41, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Maria Pérez-Vendrell, A.; Teresa Blay, M.; Terra, X.; Ardévol, A.; Pinent, M. A Specific Dose of Grape Seed-Derived Proanthocyanidins to Inhibit Body Weight Gain Limits Food Intake and Increases Energy Expenditure in Rats. Eur. J. Nutr. 2017, 56, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Yoon, Y.R.; Lee, H.J.; Yoon, S.; Kim, S.Y.; Shin, S.W.; An, J.J.; Kim, M.S.; Choi, S.Y.; Sun, W.; et al. Enhanced Hypothalamic Leptin Signaling in Mice Lacking Dopamine D2 Receptors. J. Biol. Chem. 2010, 285, 8905–8917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, A.; MacI, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of Procyanidin Dimers and Trimers and Matrix Food Effects in in Vitro and in Vivo Models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smedes, F.; Thomasen, T.K. Evaluation of the Bligh & Dyer Lipid Determination Method. Mar. Pollut. Bull. 1996, 32, 681–688. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2015, 88, 524–545. [Google Scholar] [CrossRef]
- Visser, M.E.; Caro, S.P.; van Oers, K.; Schaper, S.V.; Helm, B. Phenology, Seasonal Timing and Circannual Rhythms: Towards a Unified Framework. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3113–3127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic Compounds and Biological Rhythms: Who Takes the Lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. The Exposure to Different Photoperiods Strongly Modulates the Glucose and Lipid Metabolisms of Normoweight Fischer 344 Rats. Front. Physiol. 2018, 9, 416. [Google Scholar] [CrossRef]
- Patel, S.R.; Hu, F.B. Short Sleep Duration and Weight Gain: A Systematic Review. Obesity 2008, 16, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Heideman, P.D.; Sylvester, C.J. Reproductive Photoresponsiveness in Unmanipulated Male Fischer 344 Laboratory Rats. Biol. Reprod. 1997, 57, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Tavolaro, F.M.; Thomson, L.M.; Ross, A.W.; Morgan, P.J.; Helfer, G. Photoperiodic Effects on Seasonal Physiology, Reproductive Status and Hypothalamic Gene Expression in Young Male F344 Rats. J. Neuroendocrinol. 2015, 27, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.W.; Helfer, G.; Russell, L.; Darras, V.M.; Morgan, P.J. Thyroid Hormone Signalling Genes Are Regulated by Photoperiod in the Hypothalamus of F344 Rats. PLoS ONE 2011, 6, e21351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Kanikowska, D.; Iwase, S.; Shimizu, Y.; Nishimura, N.; Inukai, Y.; Sato, M.; Sugenoya, J. Seasonal Differences in Melatonin Concentrations and Heart Rates during Sleep in Obese Subjects in Japan. Int. J. Biometeorol. 2013, 57, 743–748. [Google Scholar] [CrossRef]
- Spady, D.K.; Woollett, L.A.; Dietschy, J.M. Regulation of Plasma LDL-Cholesterol Levels by Dietary Cholesterol and Fatty Acids. Annu. Rev. Nutr. 1993, 13, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-Q.; Liu, W.-Y.; Wu, S.-J.; Zhu, G.-Q.; Braddock, M.; Zhang, D.-C.; Shi, K.-Q.; Song, D.; Zheng, M.-H.; Sun, D.-Q.; et al. Increased Levels of Low-Density Lipoprotein Cholesterol within the Normal Range as a Risk Factor for Nonalcoholic Fatty Liver Disease. Oncotarget 2015, 7, 5728–5737. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, J.; Kumagae, G.; Sata, T.; Ikeda, M.; Tsutsumi, S.; Arakawa, K. Seasonal Variation of Serum High Density Lipoprotein Cholesterol Levels in Men. Atherosclerosis 1983, 48, 167–172. [Google Scholar] [CrossRef]
- Fager, G.; Wiklund, O.; Olofsson, S.O.; Bondjers, G. Seasonal Variations in Serum Lipid and Apolipoprotein Levels Evaluated by Periodic Regression Analyses. J. Chronic Dis. 1982, 35, 643–648. [Google Scholar] [CrossRef]
- Ma, Y.; Olendzki, B.C.; Li, W.; Hafner, A.R.; Chiriboga, D.; Hebert, J.R.; Campbell, M.; Sarnie, M.; Ockene, I.S. Seasonal Variation in Food Intake, Physical Activity, and Body Weight in a Predominantly Overweight Population. Eur. J. Clin. Nutr. 2006, 60, 519–528. [Google Scholar] [CrossRef]
- Carabaza, A.; Ciudad, C.J.; Baqué, S.; Guinovart, J.J. Glucose Has to Be Phosphorylated to Activate Glycogen Synthase, but Not to Inactivate Glycogen Phosphorylase in Hepatocytes. FEBS Lett. 1992, 296, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Kato, S.; Ichihara, A. Glucagon and Glucose as Major Regulators of Glycogen Metabolism in Primary Cultured Rat Hepatocytes. J. Biochem. 1984, 95, 1691–1696. [Google Scholar] [CrossRef]
- Stalmans, W.; de Wulf, H.; Hue, L.; Hers, H.-G. The Sequential Inactivation of Glycogen Phosphorylase and Activation of Glycogen Synthetase in Liver after the Administration of Glucose to Mice and Rats. The Mechanism of the Hepatic Threshold to Glucose. Eur. J. Biochem. 1974, 41, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Gumaa, K.A.; MacLeod, R.M.; McLean, P. The Pentose Phosphate Pathway of Glucose Metabolism. Influence of a Growth-Hormone-Secreting Pituitary Tumour on the Oxidative and Non-Oxidative Reactions of the Cycle in Liver. Biochem. J. 1969, 113, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, E.S.; Sherry, A.D.; Malloy, C.R. Interaction between the Pentose Phosphate Pathway and Gluconeogenesis from Glycerol in the Liver. J. Biol. Chem. 2014, 289, 32593–32603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockall, A.G.; Sohaib, S.A.; Evans, D.; Kaltsas, G.; Isidori, A.M.; Monson, J.P.; Besser, G.M.; Grossman, A.B.; Reznek, R.H. Hepatic Steatosis in Cushing’s Syndrome: A Radiological Assessment Using Computed Tomography. Eur. J. Endocrinol. 2003, 149, 543–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amatruda, J.M.; Livingston, J.N.; Lockwood, D.H. Cellular Mechanisms in Selected States of Insulin Resistance: Human Obesity, Glucocorticoid Excess, and Chronic Renal Failure. Diabetes/Metab. Rev. 1985, 1, 293–317. [Google Scholar] [CrossRef]
- Oleshchuk, O.; Ivankiv, Y.; Falfushynska, H.; Mudra, A.; Lisnychuk, N. Hepatoprotective Effect of Melatonin in Toxic Liver Injury in Rats. Medicina 2019, 55, 304. [Google Scholar] [CrossRef] [Green Version]
- Bonomini, F.; Borsani, E.; Favero, G.; Rodella, L.F.; Rezzani, R. Dietary Melatonin Supplementation Could Be a Promising Preventing/Therapeutic Approach for a Variety of Liver Diseases. Nutrients 2018, 10, 1135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-J.; Meng, X.; Li, Y.; Zhou, Y.; Xu, D.-P.; Li, S.; Li, H.-B. Molecular Sciences Effects of Melatonin on Liver Injuries and Diseases. Int. J. Mol. Sci. 2017, 18, 673. [Google Scholar] [CrossRef] [Green Version]
- Ohta, Y.; Kongo, M.; Sasaki, E.; Nishida, K.; Ishiguro, I. Therapeutic Effect of Melatonin on Carbon Tetrachloride-Induced Acute Liver Injury in Rats. J. Pineal Res. 2000, 28, 119–126. [Google Scholar] [CrossRef]
- Tahan, G.; Akin, H.; Aydogan, F.; Ramadan, S.S.; Yapicier, O.; Tarcin, O.; Uzun, H.; Tahan, V.; Zengin, K. Melatonin Ameliorates Liver Fibrosis Induced by Bile-Duct Ligation in Rats. Can. J. Surg. 2010, 53, 313. [Google Scholar]
- Chojnacki, C.; Walecka-Kapica, E.; Romanowski, M.; Chojnacki, J.; Klupinska, G. Protective Role of Melatonin in Liver Damage. Curr. Pharm. Des. 2014, 20, 4828–4833. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Modulate Melatonin Levels in Plasma and the Expression Pattern of Clock Genes in the Hypothalamus of Rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Basseri, S.; Austin, R.C. ER Stress and Lipogenesis: A Slippery Slope toward Hepatic Steatosis. Dev. Cell 2008, 15, 795–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colgan, S.M.; Tang, D.; Werstuck, G.H.; Austin, R.C. Endoplasmic Reticulum Stress Causes the Activation of Sterol Regulatory Element Binding Protein-2. Int. J. Biochem. Cell Biol. 2007, 39, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.N.; Ye, J. Proteolytic Activation of Sterol Regulatory Element-Binding Protein Induced by Cellular Stress through Depletion of Insig-1. J. Biol. Chem. 2004, 279, 45257–45265. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.R.; Lee, E.J.; Shin, K.O.; Kim, M.H.; Pewzner-Jung, Y.; Lee, Y.M.; Park, J.W.; Futerman, A.H.; Park, W.J. Hepatic Triglyceride Accumulation via Endoplasmic Reticulum Stress-Induced SREBP-1 Activation Is Regulated by Ceramide Synthases. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeong, S.K.; Kim, H.R.; Kim, D.S.; Chae, S.W.; Chae, H.J. Effects of Triglyceride on ER Stress and Insulin Resistance. Biochem. Biophys. Res. Commun. 2007, 363, 140–145. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Topczewski, F.; Pagliassotti, M.J. Saturated Fatty Acids Induce Endoplasmic Reticulum Stress and Apoptosis Independently of Ceramide in Liver Cells. Am. J. Physiol. Endocrinol. Metab. 2006, 291, 275–281. [Google Scholar] [CrossRef]
- Ding, R.-B.; Bao, J.; Deng, C.-X. Emerging Roles of SIRT1 in Fatty Liver Diseases. Int. J. Biol. Sci 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Kim, H.S.; Xiao, C.; Wang, R.H.; Lahusen, T.; Xu, X.; Vassilopoulos, A.; Vazquez-Ortiz, G.; Jeong, W.-I.; Park, O.; Ki, S.H.; et al. Hepatic Specific Disruption of SIRT6 in Mice Results in Fatty Liver Formation Due to Enhanced Glycolysis and Triglyceride Synthesis. Cell Metab. 2010, 12, 224. [Google Scholar] [CrossRef] [Green Version]
- Aragonès, G.; Suárez, M.; Ardid-Ruiz, A.; Vinaixa, M.; Rodríguez, M.A.; Correig, X.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Boost Hepatic NAD + Metabolism and SIRT1 Expression and Activity in a Dose-Dependent Manner in Healthy Rats. Sci. Rep. 2016, 6, 24977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foretz, M.; Guichard, C.; Ferré, P.; Foufelle, F. Sterol Regulatory Element Binding Protein-1c Is a Major Mediator of Insulin Action on the Hepatic Expression of Glucokinase and Lipogenesis-Related Genes. Proc. Natl. Acad. Sci. USA 1999, 96, 12737–12742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dentin, R.; Pé, J.-P.; Benhamed, F.; Foufelle, F.; Ferré, P.; Ronique Fauveau, V.; Magnuson, M.A.; Girard, J.; Postic, C. Hepatic Glucokinase Is Required for the Synergistic Action of ChREBP and SREBP-1c on Glycolytic and Lipogenic Gene Expression. J. Biol. Chem. 2004, 279, 20314–20326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikegami, K.; Iigo, M.; Yoshimura, T. Circadian Clock Gene Per2 Is Not Necessary for the Photoperiodic Response in Mice. PLoS ONE 2013, 8, 58482. [Google Scholar] [CrossRef]
- Lamia, K.A.; Storch, K.-F.; Weitz, C.J. Physiological Significance of a Peripheral Tissue Circadian Clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Zhao, B.; Huang, L.; Shen, Q.; Ma, L.; Chen, Y.; Wu, T.; Fu, Z. Effects of Altered Photoperiod on Circadian Clock and Lipid Metabolism in Rats. Chronobiol. Int. 2017, 34, 1094–1104. [Google Scholar] [CrossRef]








| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| Acacα | TGCAGGTATCCCCACTCTTC | TTCTGATTCCCTTCCCTCCT |
| Atf4 | TATGGATGGGTTGGTCAGTG | CTCATCTGGCATGGTTTCC |
| Atf6 | GACTGGGAGTCCACGTTGTT | GAACAGGAGTCTGTGGACCG |
| Bmal1 | GTAGATCAGAGGGCGACGGCTA | CTTGTCTGTAAAACTTGCCTGTGAC |
| Cd36 | GTCCTGGCTGTGTTTGGA | GCTCAAAGATGGCTCCATTG |
| Chop | AAGATGAGCGGGTGGCAGCG | CCGGTTTCTGCTTTCAGGTGTGGT |
| Cry1 | TGGAAGGTATGCGTGTCCTC | TCCAGGAGAACCTCCTCACG |
| Fatp5 | CCTGCCAAGCTTCGTGCTAAT | GCTCATGTGATAGGATGGCTGG |
| G6pdh | ACCAGGCATTCAAAACGCAT | CAGTCTCAGGGAAGTGTGGT |
| Gk | CTGTGAAAGCGTGTCCACTC | GCCCTCCTCTGATTCGATGA |
| Grp78 | CTACGAAGGTGAACGACCCC | ATTTCTTCAGGGGTCAGGCG |
| Nampt | CTCTTCACAAGAGACTGCCG | TTCATGGTCTTTCCCCCACG |
| Nr1d1 | ACAGCTGACACCACCCAGATC | CATGGGCATAGGTGAAGATTTCT |
| Per2 | CGGACCTGGCTTCAGTTCAT | AGGATCCAAGAACGGCACAG |
| Pparα | CGGCGTTGAAAACAAGGAGG | TTGGGTTCCATGATGTCGCA |
| Ppia | CCAAACACAAATGGTTCCCAGT | ATTCCTGGACCCAAAACGCT |
| Rorα | CCCGATGTCTTCAAATCCTTAGG | TCAGTCAGATGCATAGAACACAAACTC |
| Srebp1c | CCCACCCCCTTACACACC | GCCTGCGGTCTTCATTGT |
| s-Xbp1 | AAACAGAGTAGCAGCACAGACTGC | TCCTTCTGGGTAGACCTCTGGGAG |
| Serum Parameters | L12 | L18 | L6 | 2wA | |||
|---|---|---|---|---|---|---|---|
| STD-VH | STD-GSPE | STD-VH | STD-GSPE | STD-VH | STD-GSPE | ||
| Glucose (mg/dL) | 110.96 ± 4.67 a | 102.72 ± 2.63 a | 110.34 ± 2.86 a | 141.81 ± 3.06 b | 136.34 ± 5.82 b | 154.49 ± 6.65 c | T, P, T*P |
| Cholesterol (mg/dL) | 98.53 ± 5.30 a | 104.54 ± 6.98 ab | 107.15 ± 7.06 ab | 108.36 ± 4.84 ab | 98.60 ± 3.61 a | 117.47 ± 4.15 b | T |
| Triglycerides (mg/dL) | 56.13 ± 4.08 ab | 46.73 ± 2.02 b | 58.72 ± 4.69 ab | 60.71 ± 5.84 ab,* | 52.90 ± 3.25 ab | 64.73 ± 6.93 a | |
| HDL (mg/dL) | 35.33 ± 1.43 a | 32.11 ± 2.28 ab | 28.57 ± 2.25 bc | 31.38 ± 1.13 abd | 25.44 ± 1.27 c | 26.93 ± 2.49 cd | P |
| LDL (mg/dL) | 30.55 ± 3.20 a | 34.79 ± 2.59 a | 32.47 ± 3.33 a | 20.80 ± 1.94 b | 19.45 ± 1.47 b | 19.43 ± 2.08 b | P, T*P |
| Parameters | L12 | L18 | L6 | 2wA | |||
|---|---|---|---|---|---|---|---|
| STD-VH | STD-GSPE | STD-VH | STD-GSPE | STD-VH | STD-GSPE | ||
| Cholesterol (mg/g) | 1.19 ± 0.06 ab | 1.27 ± 0.08 a | 1.04 ± 0.07 b | 1.09 ± 0.07 ab | 1.14 ± 0.05 ab | 1.12 ± 0.06 ab | |
| Triglycerides (mg/g) | 2.62 ± 0.17 b | 2.98 ± 0.10 ab | 3.44 ± 0.35 abc | 3.34 ± 0.13 a | 3.68 ± 0.29 ac | 3.84 ± 0.15 c | P |
| Phospholipids (mg/g) | 6.82 ± 0.27 a | 6.86 ± 0.36 a | 6.3 ± 0.34 a | 6.32 ± 0.26 a | 6.92 ± 0.34 a | 7.05 ± 0.42 a | |
| Liver weight (g) | 14.78 ± 0.5 a | 15.25 ± 0.6 a | 15.21 ± 0.7 a | 15.13 ± 0.61 a | 15.6 ± 0.47 a | 14.68 ± 0.61 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, R.M.; Colom-Pellicer, M.; Blanco, J.; Calvo, E.; Aragonès, G.; Mulero, M. Grape-Seed Procyanidin Extract (GSPE) Seasonal-Dependent Modulation of Glucose and Lipid Metabolism in the Liver of Healthy F344 Rats. Biomolecules 2022, 12, 839. https://0-doi-org.brum.beds.ac.uk/10.3390/biom12060839
Rodríguez RM, Colom-Pellicer M, Blanco J, Calvo E, Aragonès G, Mulero M. Grape-Seed Procyanidin Extract (GSPE) Seasonal-Dependent Modulation of Glucose and Lipid Metabolism in the Liver of Healthy F344 Rats. Biomolecules. 2022; 12(6):839. https://0-doi-org.brum.beds.ac.uk/10.3390/biom12060839
Chicago/Turabian StyleRodríguez, Romina M., Marina Colom-Pellicer, Jordi Blanco, Enrique Calvo, Gerard Aragonès, and Miquel Mulero. 2022. "Grape-Seed Procyanidin Extract (GSPE) Seasonal-Dependent Modulation of Glucose and Lipid Metabolism in the Liver of Healthy F344 Rats" Biomolecules 12, no. 6: 839. https://0-doi-org.brum.beds.ac.uk/10.3390/biom12060839