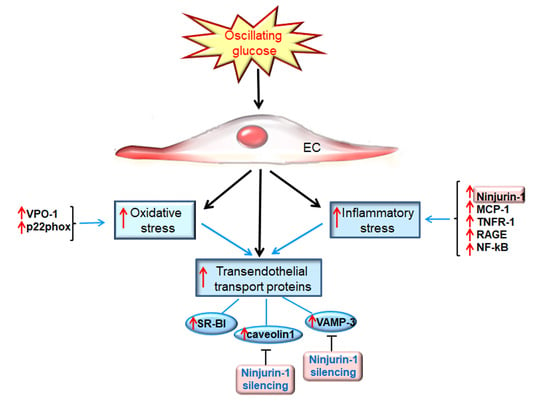
Oscillating Glucose Induces the Increase in Inflammatory Stress through Ninjurin-1 Up-Regulation and Stimulation of Transport Proteins in Human Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Experimental Design
2.3. In Vitro Silencing of Ninjurin-1 in OG-Exposed EC
2.4. RNA Isolation and Gene Expression Measurement
2.5. Quantification of Protein Expression in Cell Lysates
2.6. Measurement of Secreted MCP-1 in the Cell Culture Medium
2.7. Monocyte Adhesion Assay
2.8. Intracellular ROS Level Measurements
2.9. Statistical Analysis
3. Results
3.1. Oscillating Glucose Up-Regulates Endothelial Expression of Ninj-1 and MCP-1, Promoting Increased Adhesion of Monocytes to ECs from EA.hy.926 Cell Line
3.2. Oscillating Glucose Up-Regulates the Expression of TNFR1 and RAGE in ECs from EA.hy926 Cell Line
3.3. Oscillating Glucose and Constant High Glucose Stimulate the Proteins Involved in the Regulation of Transendothelial Transport
3.4. Oscillating Glucose Determines Increased ROS Production and Endoplasmic Reticulum Stress in ECs from EA.hy926 Cell Line
3.5. Oscillating Glucose Activates the Pro-Inflammatory NF-kB Transcription Factor and p38 MAPK
3.6. Oscillating Glucose Stimulates Inflammatory Stress by Inducing Oxidative Stress and NF-kB Transcription Factor in ECs from EA.hy926 Line
3.7. Oxidative Stress and NF-kB Modulate the Expression of Proteins Involved in Transendothelial Transport
3.8. Ninjurin 1 Silencing Decreases the Expression of Proteins Involved in Transendothelial Transport
3.9. OG Stimulates the Transendothelial Transport Proteins in Primary EC Partially through Mechanisms Involving Ninj-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Lv, L.; Yang, Y.; Chen, D.; Liu, G.; Chen, L.; Song, Y.; He, L.; Li, X.; Tian, H.; et al. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin. Endocrinol. 2012, 76, 810–815. [Google Scholar] [CrossRef]
- Sezer, H.; Yazici, D.; Copur, S.; Dagel, T.; Deyneli, O.; Kanbay, M. The relationship between glycemic variability and blood pressure variability in normoglycemic normotensive individuals. Blood Press. Monit. 2021, 26, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, J.; Skov, M.W.; Kanters, J.K.; Lind, B.; Isaksen, J.L.; Blanche, P.; Haunso, S.; Kober, L.; Svendsen, J.H.; Olesen, M.S.; et al. Visit-to-Visit Variability of Hemoglobin A1c in People Without Diabetes and Risk of Major Adverse Cardiovascular Events and All-Cause Mortality. Diabetes Care 2019, 42, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Kesavadev, J.; Misra, A.; Saboo, B.; Aravind, S.R.; Hussain, A.; Czupryniak, L.; Raz, I. Blood glucose levels should be considered as a new vital sign indicative of prognosis during hospitalization. Diabetes Metab. Syndr. 2021, 15, 221–227. [Google Scholar] [CrossRef]
- Zhou, J.J.; Koska, J.; Bahn, G.; Reaven, P. Fasting Glucose Variation Predicts Microvascular Risk in ACCORD and VADT. J. Clin. Endocrinol. Metab. 2020, 106, e1150–e1162. [Google Scholar] [CrossRef]
- Scott, E.S.; Januszewski, A.S.; O’Connell, R.; Fulcher, G.; Scott, R.; Kesaniemi, A.; Wu, L.; Colagiuri, S.; Keech, A.; Jenkins, A.J. Long-Term Glycemic Variability and Vascular Complications in Type 2 Diabetes: Post Hoc Analysis of the FIELD Study. J. Clin. Endocrinol. Metab. 2020, 105, e3638–e3649. [Google Scholar] [CrossRef]
- Sun, B.; He, F.; Gao, Y.; Zhou, J.; Sun, L.; Liu, R.; Xu, H.; Chen, X.; Zhou, H.; Liu, Z.; et al. Prognostic impact of visit-to-visit glycemic variability on the risks of major adverse cardiovascular outcomes and hypoglycemia in patients with different glycemic control and type 2 diabetes. Endocrine 2019, 64, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Miao, L.F.; Qian, L.L.; Wang, N.; Qi, M.M.; Zhang, Y.M.; Dang, S.P.; Wu, Y.; Wang, R.X. Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications. Front. Endocrinol. 2019, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Luo, Z.; Zhou, J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc. Diabetol. 2021, 20, 9. [Google Scholar] [CrossRef]
- Wakasugi, S.; Mita, T.; Katakami, N.; Okada, Y.; Yoshii, H.; Osonoi, T.; Kuribayashi, N.; Taneda, Y.; Kojima, Y.; Gosho, M.; et al. Associations between continuous glucose monitoring-derived metrics and arterial stiffness in Japanese patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.D.; Shen, Y.; Ding, F.H.; Yang, Z.K.; Hu, J.; Shen, W.F.; Zhang, R.Y.; Lu, L.; Wang, X.Q. Visit-to-visit fasting plasma glucose variability is associated with left ventricular adverse remodeling in diabetic patients with STEMI. Cardiovasc. Diabetol. 2020, 19, 131. [Google Scholar] [CrossRef]
- Li, S.; Tang, X.; Luo, Y.; Wu, B.; Huang, Z.; Li, Z.; Peng, L.; Ling, Y.; Zhu, J.; Zhong, J.; et al. Impact of long-term glucose variability on coronary atherosclerosis progression in patients with type 2 diabetes: A 2.3 year follow-up study. Cardiovasc. Diabetol. 2020, 19, 146. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Han, K.; Park, S.; Yu, J.H.; Seo, J.A.; Kim, N.H.; Yoo, H.J.; Kim, S.G.; Choi, K.M.; Baik, S.H.; et al. Glucose variability and the risks of stroke, myocardial infarction, and all-cause mortality in individuals with diabetes: Retrospective cohort study. Cardiovasc. Diabetol. 2020, 19, 144. [Google Scholar] [CrossRef]
- Del Olmo Garcia, M.I.; Merino-Torres, J.F. GLP 1 receptor agonists, glycemic variability, oxidative stress and acute coronary syndrome. Med. Hypotheses 2020, 136, 109504. [Google Scholar] [CrossRef]
- Kaze, A.D.; Santhanam, P.; Erqou, S.; Ahima, R.S.; Echouffo-Tcheugui, J.B. Long-term variability of glycemic markers and risk of all-cause mortality in type 2 diabetes: The Look AHEAD study. BMJ Open Diabetes Res. Care 2020, 8, e001753. [Google Scholar] [CrossRef]
- Cheng, P.C.; Kao, C.H. Postprandial plasma glucose excursion is associated with an atherogenic lipid profile in individuals with type 2 diabetes mellitus: A cross-sectional study. PLoS ONE 2021, 16, e0258771. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Li, T.C.; Li, C.I.; Liu, C.S.; Lin, W.Y.; Yang, S.Y.; Chiang, J.H.; Huang, C.C.; Sung, F.C.; Lin, C.C. Visit-to-Visit Glucose Variability Predicts the Development of End-Stage Renal Disease in Type 2 Diabetes: 10-Year Follow-Up of Taiwan Diabetes Study. Medicine 2015, 94, e1804. [Google Scholar] [CrossRef]
- Picconi, F.; Parravano, M.; Ylli, D.; Pasqualetti, P.; Coluzzi, S.; Giordani, I.; Malandrucco, I.; Lauro, D.; Scarinci, F.; Giorno, P.; et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: The role of glycemic variability. Acta Diabetol. 2017, 54, 489–497. [Google Scholar] [CrossRef]
- Kwai, N.C.; Arnold, R.; Poynten, A.M.; Krishnan, A.V. Association between glycemic variability and peripheral nerve dysfunction in type 1 diabetes. Muscle Nerve 2016, 54, 967–969. [Google Scholar] [CrossRef]
- Kohnert, K.D.; Freyse, E.J.; Salzsieder, E. Glycaemic variability and pancreatic β-cell dysfunction. Curr. Diabetes Rev. 2012, 8, 345–354. [Google Scholar] [CrossRef]
- Murata, M.; Adachi, H.; Oshima, S.; Kurabayashi, M. Glucose fluctuation and the resultant endothelial injury are correlated with pancreatic β cell dysfunction in patients with coronary artery disease. Diabetes Res. Clin. Pract. 2017, 131, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Anno, T.; Kawasaki, F.; Kimura, T.; Hirukawa, H.; Mune, T.; Okimoto, N.; Kaku, K.; Kaneto, H. Association of the Glycemic Fluctuation as well as Glycemic Control with the Pancreatic β-cell Function in Japanese Subjects with Type 2 Diabetes Mellitus. Intern. Med. 2019, 58, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, C.; Liu, T.; Ling, H.; Cheng, M.; Zhou, X.; Wang, S.; Mao, Y.; Chen, L.; Zhang, R.; Li, W. Glucose variability aggravates cardiac fibrosis by altering AKT signalling path. Diab. Vasc. Dis. Res. 2017, 14, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Teshima, Y.; Fukui, A.; Kondo, H.; Nishio, S.; Nakagawa, M.; Saikawa, T.; Takahashi, N. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc. Res. 2014, 104, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Guo, J.; Kang, Y.; Ou, X. Mismatch between GLUTs and glucose levels causes neuronal damage during glucose fluctuations. Med. Hypotheses 2018, 112, 37–39. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Saik, O.V.; Korbut, A.I. Glucose Variability: How Does It Work? Int. J. Mol. Sci. 2021, 22, 7783. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Emoto, M.; Abe, M.; Inaba, M. Visualization of Blood Glucose Fluctuations Using Continuous Glucose Monitoring in Patients Undergoing Hemodialysis. J. Diabetes Sci. Technol. 2019, 13, 413–414. [Google Scholar] [CrossRef] [Green Version]
- Mian, Z.; Hermayer, K.L.; Jenkins, A. Continuous Glucose Monitoring: Review of an Innovation in Diabetes Management. Am. J. Med. Sci. 2019, 358, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Forsblom, C.; Harjutsalo, V.; Groop, P.H. Dietary carbohydrate intake and cardio-metabolic risk factors in type 1 diabetes. Diabetes Res. Clin. Pract. 2019, 155, 107818. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.R.; Francois, M.E.; Little, J.P. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am. J. Clin. Nutr. 2019, 109, 1302–1309. [Google Scholar] [CrossRef]
- Farabi, S.S.; Carley, D.W.; Smith, D.; Quinn, L. Impact of exercise on diurnal and nocturnal markers of glycaemic variability and oxidative stress in obese individuals with type 2 diabetes or impaired glucose tolerance. Diab. Vasc. Dis. Res. 2015, 12, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira, F.R.; Umpierre, D.; Bock, P.M.; Waclawovsky, G.; Guerra, A.P.; Donelli, A.; Andrades, M.; Casali, K.R.; Schaan, B.D. Effect of exercise on glucose variability in healthy subjects: Randomized crossover trial. Biol. Sport. 2019, 36, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Kim, J.H. Glycemic Variability: How Do We Measure It and Why Is It Important? Diabetes Metab. J. 2015, 39, 273–282. [Google Scholar] [CrossRef]
- Frontoni, S.; Di Bartolo, P.; Avogaro, A.; Bosi, E.; Paolisso, G.; Ceriello, A. Glucose variability: An emerging target for the treatment of diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 102, 86–95. [Google Scholar] [CrossRef]
- Famulla, S.; Pieber, T.R.; Eilbracht, J.; Neubacher, D.; Soleymanlou, N.; Woerle, H.J.; Broedl, U.C.; Kaspers, S. Glucose Exposure and Variability with Empagliflozin as Adjunct to Insulin in Patients with Type 1 Diabetes: Continuous Glucose Monitoring Data from a 4-Week, Randomized, Placebo-Controlled Trial (EASE-1). Diabetes Technol. Ther. 2017, 19, 49–60. [Google Scholar] [CrossRef]
- Suzuki, D.; Yamada, H.; Yoshida, M.; Funazaki, S.; Amamoto, M.; Morimoto, J.; Hara, K. Sodium-glucose cotransporter 2 inhibitors improved time-in-range without increasing hypoglycemia in Japanese patients with type 1 diabetes: A retrospective, single-center, pilot study. J. Diabetes Investig. 2020, 11, 1230–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toma, L.; Stancu, C.S.; Sima, A.V. Endothelial Dysfunction in Diabetes Is Aggravated by Glycated Lipoproteins; Novel Molecular Therapies. Biomedicines 2020, 9, 18. [Google Scholar] [CrossRef]
- Maeda, M.; Hayashi, T.; Mizuno, N.; Hattori, Y.; Kuzuya, M. Intermittent high glucose implements stress-induced senescence in human vascular endothelial cells: Role of superoxide production by NADPH oxidase. PLoS ONE 2015, 10, e0123169. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gong, J.; Chen, Y.; Jiang, S. Periodic vs. constant high glucose in inducing pro-inflammatory cytokine expression in human coronary artery endothelial cells. Inflamm. Res. 2013, 62, 697–701. [Google Scholar] [CrossRef]
- Ren, Y.; Tao, S.; Zheng, S.; Zhao, M.; Zhu, Y.; Yang, J.; Wu, Y. Salvianolic acid B improves vascular endothelial function in diabetic rats with blood glucose fluctuations via suppression of endothelial cell apoptosis. Eur. J. Pharmacol. 2016, 791, 308–315. [Google Scholar] [CrossRef]
- Liu, T.S.; Pei, Y.H.; Peng, Y.P.; Chen, J.; Jiang, S.S.; Gong, J.B. Oscillating high glucose enhances oxidative stress and apoptosis in human coronary artery endothelial cells. J. Endocrinol. Invest. 2014, 37, 645–651. [Google Scholar] [CrossRef]
- Wang, X.; Qin, J.; Zhang, X.; Peng, Z.; Ye, K.; Wu, X.; Yang, X.; Shi, H.; Zhao, Z.; Guo, X.; et al. Functional blocking of Ninjurin1 as a strategy for protecting endothelial cells in diabetes mellitus. Clin. Sci. 2018, 132, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, T.K.; Jeong, S.J.; Jung, I.H.; Kim, N.; Lee, M.N.; Sonn, S.K.; Seo, S.; Jin, J.; Kweon, H.Y.; et al. Anti-Inflammatory Actions of Soluble Ninjurin-1 Ameliorate Atherosclerosis. Circulation 2020, 142, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kim, I.D.; Lee, H.; Luo, L.; Kim, S.W.; Lee, J.K. Neuroprotective and Anti-inflammatory Effects of a Dodecamer Peptide Harboring Ninjurin 1 Cell Adhesion Motif in the Postischemic Brain. Mol. Neurobiol. 2018, 55, 6094–6111. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, Y.; Li, H. The Role of Ninjurin1 and Its Impact beyond the Nervous System. Dev. Neurosci. 2020, 42, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.N.; Choi, M.J.; Kim, W.J.; Kwon, M.H.; Song, K.M.; Park, J.M.; Das, N.D.; Kwon, K.D.; Batbold, D.; Oh, G.T.; et al. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc. Natl. Acad. Sci. USA 2014, 111, E2731–E2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayagaki, N.; Kornfeld, O.S.; Lee, B.L.; Stowe, I.B.; O’Rourke, K.; Li, Q.; Sandoval, W.; Yan, D.; Kang, J.; Xu, M.; et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 2021, 591, 131–136. [Google Scholar] [CrossRef]
- Piconi, L.; Quagliaro, L.; Assaloni, R.; Da Ros, R.; Maier, A.; Zuodar, G.; Ceriello, A. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab. Res. Rev. 2006, 22, 198–203. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Toma, L.; Sanda, G.M.; Raileanu, M.; Stancu, C.S.; Niculescu, L.S.; Sima, A.V. Ninjurin-1 upregulated by TNFα receptor 1 stimulates monocyte adhesion to human TNFα-activated endothelial cells; benefic effects of amlodipine. Life Sci. 2020, 249, 117518. [Google Scholar] [CrossRef]
- Toma, L.; Sanda, G.M.; Deleanu, M.; Stancu, C.S.; Sima, A.V. Glycated LDL increase VCAM-1 expression and secretion in endothelial cells and promote monocyte adhesion through mechanisms involving endoplasmic reticulum stress. Mol. Cell. Biochem. 2016, 417, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Owens, D. Glucose variability and diabetes complications: Risk factor or biomarker? Can we disentangle the “Gordian Knot”? Diabetes Metab. 2021, 47, 101225. [Google Scholar] [CrossRef]
- Hoffman, R.P.; Dye, A.S.; Huang, H.; Bauer, J.A. Effects of glucose control and variability on endothelial function and repair in adolescents with type 1 diabetes. ISRN Endocrinol. 2013, 2013, 876547. [Google Scholar] [CrossRef] [Green Version]
- Jennewein, C.; Sowa, R.; Faber, A.C.; Dildey, M.; von Knethen, A.; Meybohm, P.; Scheller, B.; Dröse, S.; Zacharowski, K. Contribution of Ninjurin1 to Toll-like receptor 4 signaling and systemic inflammation. Am. J. Respir. Cell Mol. Biol. 2015, 53, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Zhang, J.; Yan, W.; Cho, S.J.; Lucchesi, C.; Chen, M.; Huang, E.C.; Scoumanne, A.; Zhang, W.; Chen, X. Ninjurin 1 has two opposing functions in tumorigenesis in a p53-dependent manner. Proc. Natl. Acad. Sci. USA 2017, 114, 11500–11505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Da Ros, R.; Maier, A.; Zuodar, G.; Ceriello, A. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: The distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 2005, 183, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Piconi, L.; Quagliaro, L.; Da Ros, R.; Assaloni, R.; Giugliano, D.; Esposito, K.; Szabo, C.; Ceriello, A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: The role of poly(ADP-ribose) polymerase. J. Thromb. Haemost. 2004, 2, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Azuma, K.; Iesaki, T.; Sato, F.; Hirose, T.; Shimizu, T.; Tanaka, Y.; Daida, H.; Kawamori, R.; Watada, H. Temporary hyperglycaemia provokes monocyte adhesion to endothelial cells in rat thoracic aorta. Diabetologia 2005, 48, 2667–2674. [Google Scholar] [CrossRef] [Green Version]
- Watada, H.; Azuma, K.; Kawamori, R. Glucose fluctuation on the progression of diabetic macroangiopathy—New findings from monocyte adhesion to endothelial cells. Diabetes Res. Clin. Pract. 2007, 77 (Suppl. S1), S58–S61. [Google Scholar] [CrossRef]
- Jang, E.; Robert, J.; Rohrer, L.; von Eckardstein, A.; Lee, W.L. Transendothelial transport of lipoproteins. Atherosclerosis 2020, 315, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Schubert, W.; Frank, P.G.; Razani, B.; Park, D.S.; Chow, C.W.; Lisanti, M.P. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 2001, 276, 48619–48622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, F.; Yang, X.; Zhou, F.; Wu, P.H.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zhang, Y.; et al. C-reactive protein promotes atherosclerosis by increasing LDL transcytosis across endothelial cells. Br. J. Pharmacol. 2014, 171, 2671–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes. Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Maamoun, H.; Zachariah, M.; McVey, J.H.; Green, F.R.; Agouni, A. Heme oxygenase (HO)-1 induction prevents Endoplasmic Reticulum stress-mediated endothelial cell death and impaired angiogenic capacity. Biochem. Pharmacol. 2017, 127, 46–59. [Google Scholar] [CrossRef]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriello, A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: The role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef] [Green Version]
- Maamoun, H.; Benameur, T.; Pintus, G.; Munusamy, S.; Agouni, A. Crosstalk Between Oxidative Stress and Endoplasmic Reticulum (ER) Stress in Endothelial Dysfunction and Aberrant Angiogenesis Associated With Diabetes: A Focus on the Protective Roles of Heme Oxygenase (HO)-1. Front. Physiol. 2019, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Rolski, F.; Blyszczuk, P. Complexity of TNF-alpha Signaling in Heart Disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Stancu, C.S.; Sima, A.V. Caffeic acid attenuates the inflammatory stress induced by glycated LDL in human endothelial cells by mechanisms involving inhibition of AGE-receptor, oxidative, and endoplasmic reticulum stress. Biofactors 2017, 43, 685–697. [Google Scholar] [CrossRef]
- Li, J.; Schmidt, A.M. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J. Biol. Chem. 1997, 272, 16498–16506. [Google Scholar] [CrossRef] [Green Version]
- Deregowski, V.; Delhalle, S.; Benoit, V.; Bours, V.; Merville, M.P. Identification of cytokine-induced nuclear factor-kappaB target genes in ovarian and breast cancer cells. Biochem. Pharmacol. 2002, 64, 873–881. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Kraakman, M.J.; Flynn, M.C.; Nagareddy, P.R.; Schalkwijk, C.G.; Murphy, A.J. Postprandial Glucose Spikes, an Important Contributor to Cardiovascular Disease in Diabetes? Front. Cardiovasc. Med. 2020, 7, 570553. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Postprandial blood glucose. Diabetes Care 2001, 24, 775–778. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Gao, D.; Zhang, W.; Liu, S.; Yang, S.; Li, X. Puerarin suppresses high glucose-induced MCP-1 expression via modulating histone methylation in cultured endothelial cells. Life Sci. 2015, 130, 103–107. [Google Scholar] [CrossRef]
- Koziel, A.; Sobieraj, I.; Jarmuszkiewicz, W. Increased activity of mitochondrial uncoupling protein 2 improves stress resistance in cultured endothelial cells exposed in vitro to high glucose levels. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H147–H156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Zhang, X.; Ge, X.; He, C.; Liu, S.; Yang, S.; Huang, C. N-Acetylcysteine protects against cobalt chloride-induced endothelial dysfunction by enhancing glucose-6-phosphate dehydrogenase activity. FEBS Open Bio. 2022, 12, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Fiorello, M.L.; Treweeke, A.T.; Macfarlane, D.P.; Megson, I.L. The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes. Sci. Rep. 2020, 10, 19547. [Google Scholar] [CrossRef]
- Qiu, Y.; Du, B.; Xie, F.; Cai, W.; Liu, Y.; Li, Y.; Feng, L.; Qiu, L. Vaccarin attenuates high glucose-induced human EA•hy926 endothelial cell injury through inhibition of Notch signaling. Mol. Med. Rep. 2016, 13, 2143–2150. [Google Scholar] [CrossRef] [Green Version]
- Sanda, G.M.; Toma, L.; Barbalata, T.; Moraru, O.E.; Niculescu, L.S.; Sima, A.V.; Stancu, C.S. Clusterin, paraoxonase 1, and myeloperoxidase alterations induce high-density lipoproteins dysfunction and contribute to peripheral artery disease; aggravation by type 2 diabetes mellitus. Biofactors 2022, 48, 454–468. [Google Scholar] [CrossRef]
- Ghaffari, S.; Jang, E.; Naderinabi, F.; Sanwal, R.; Khosraviani, N.; Wang, C.; Steinberg, B.E.; Goldenberg, N.M.; Ikeda, J.; Lee, W.L. Endothelial HMGB1 Is a Critical Regulator of LDL Transcytosis via an SREBP2-SR-BI Axis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 200–216. [Google Scholar] [CrossRef]
- Ceriello, A. Hypothesis: The “metabolic memory”, the new challenge of diabetes. Diabetes Res. Clin. Pract. 2009, 86 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, L.; Sanda, G.M.; Stancu, C.S.; Niculescu, L.S.; Raileanu, M.; Sima, A.V. Oscillating Glucose Induces the Increase in Inflammatory Stress through Ninjurin-1 Up-Regulation and Stimulation of Transport Proteins in Human Endothelial Cells. Biomolecules 2023, 13, 626. https://0-doi-org.brum.beds.ac.uk/10.3390/biom13040626
Toma L, Sanda GM, Stancu CS, Niculescu LS, Raileanu M, Sima AV. Oscillating Glucose Induces the Increase in Inflammatory Stress through Ninjurin-1 Up-Regulation and Stimulation of Transport Proteins in Human Endothelial Cells. Biomolecules. 2023; 13(4):626. https://0-doi-org.brum.beds.ac.uk/10.3390/biom13040626
Chicago/Turabian StyleToma, Laura, Gabriela M. Sanda, Camelia S. Stancu, Loredan S. Niculescu, Mina Raileanu, and Anca V. Sima. 2023. "Oscillating Glucose Induces the Increase in Inflammatory Stress through Ninjurin-1 Up-Regulation and Stimulation of Transport Proteins in Human Endothelial Cells" Biomolecules 13, no. 4: 626. https://0-doi-org.brum.beds.ac.uk/10.3390/biom13040626