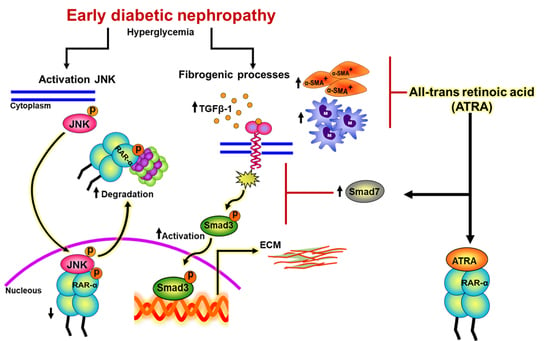
All-Trans Retinoic Acid Attenuates Fibrotic Processes by Downregulating TGF-β1/Smad3 in Early Diabetic Nephropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Experimental Diabetic Nephropathy
2.4. Experimental Design
2.5. Measurement of Renal Function and Biochemical Markers
2.6. Histopathology of the Kidney
2.7. Isolation of Glomeruli
2.8. Isolation of Proximal Tubules
2.9. Western Blot Analysis
2.10. Immunohistochemistry Analyses
2.11. Immunoprecipitation (IP)
2.12. Data Analysis
3. Results
3.1. ATRA Preserves Renal Function of Rats with Early DN
3.2. The effect of ATRA on Pathological Lesion of Early DN
3.3. ATRA Treatment Prevents Renal Accumulation of Macrophages into Glomerular and Tubule-Interstitial Areas during Initiation of DN
3.4. ATRA Treatment Decreases Profibrotic Processes during Early DN
3.5. ATRA Treatment Prevents Diabetic-Induced Changes associated to the Expression of Markers of ECM Accumulation
3.6. ATRA Treatment Ameliorates Fibrogenesis by Suppressing TGF-β1/Smad3 Signaling Pathway in Glomeruli from Diabetic Rats
3.7. ATRA Treatment Attenuates Diabetes-induced Activity of TGF-β1/Smad3 Signaling in Proximal Tubule
3.8. ATRA Inhibits Diabetes-Induced Phosphorylation and Loss of RAR-α Expression by JNK.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lv, W.; Booz, G.W.; Wang, Y.; Fan, F.; Roman, R.J. Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 2018, 820, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Q.; Shao, X.; Mou, S.; Gu, L.; Wang, L.; Zhang, Z.; Shen, J.; Zhou, Y.; Qi, C.; et al. NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp. Cell Res. 2019, 111488. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Wang, S.J.; Li, X.Q.; Shen, Y.L.; Lu, J.R.; Tian, X.H.; Rahman, K.; Zhang, L.J.; Nian, H.; Zhang, H. CXCL6 Promotes Renal Interstitial Fibrosis in Diabetic Nephropathy by Activating JAK/STAT3 Signaling Pathway. Front. Pharmacol. 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Falke, L.L.; Gholizadeh, S.; Goldschmeding, R.; Kok, R.J.; Nguyen, T.Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol. 2015, 11, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.; Lu, D.W.; Zhao, H.; Feng, Y.L.; Chen, H.; Chen, D.Q.; Vaziri, N.D.; Zhao, Y.Y. Central role of dysregulation of TGF-beta/Smad in CKD progression and potential targets of its treatment. Biomed. Pharmacother. 2018, 101, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.; Chai, Z. Transforming growth factor beta (TGFbeta) and related molecules in chronic kidney disease (CKD). Clin. Sci. 2019, 133, 287–313. [Google Scholar] [CrossRef]
- Tang, P.M.; Zhang, Y.Y.; Mak, T.S.; Tang, P.C.; Huang, X.R.; Lan, H.Y. Transforming growth factor-beta signalling in renal fibrosis: from Smads to non-coding RNAs. J. Physiol. 2018, 596, 3493–3503. [Google Scholar] [CrossRef]
- Ma, T.T.; Meng, X.M. TGF-beta/Smad and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 347–364. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, X.R.; Wang, W.; Li, J.H.; Heuchel, R.L.; Chung, A.C.; Lan, H.Y. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes 2011, 60, 590–601. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.M.; Huang, X.R.; Lan, H.Y. The preventive and therapeutic implication for renal fibrosis by targetting TGF-beta/Smad3 signaling. Clin. Sci. 2018, 132, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Mallipattu, S.K.; He, J.C. The beneficial role of retinoids in glomerular disease. Front. Med. 2015, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Yoo, B.S.; Nozaki, Y.; Sugiyama, M.; Ikoma, S.; Ohno, M.; Funauchi, M.; Kanamaru, A. Retinoic acid reduces autoimmune renal injury and increases survival in NZB/W F1 mice. J. Immunol. 2003, 170, 5793–5798. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Van, Y.H.; Kuo, C.H.; Lin, M.Y.; Liu, Y.H.; Chang, H.Y. Prevention and Reversal of Diabetes by All-Trans Retinoid Acid and Exendin-4 in NOD Mice. Int. J. Endocrinol. 2014, 2014, 435481. [Google Scholar] [CrossRef]
- Perez, A.; Ramirez-Ramos, M.; Calleja, C.; Martin, D.; Namorado, M.C.; Sierra, G.; Ramirez-Ramos, M.E.; Paniagua, R.; Sanchez, Y.; Arreola, L.; et al. Beneficial effect of retinoic acid on the outcome of experimental acute renal failure. Nephrol. Dial. Transplant. 2004, 19, 2464–2471. [Google Scholar] [CrossRef] [Green Version]
- Molina-Jijon, E.; Rodriguez-Munoz, R.; Namorado Mdel, C.; Bautista-Garcia, P.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Reyes, J.L. All-trans retinoic acid prevents oxidative stress-induced loss of renal tight junction proteins in type-1 diabetic model. J. Nutr. Biochem. 2015, 26, 441–454. [Google Scholar] [CrossRef]
- Sierra-Mondragon, E.; Molina-Jijon, E.; Namorado-Tonix, C.; Rodriguez-Munoz, R.; Pedraza-Chaverri, J.; Reyes, J.L. All-trans retinoic acid ameliorates inflammatory response mediated by TLR4/NF-kappaB during initiation of diabetic nephropathy. J. Nutr. Biochem. 2018, 60, 47–60. [Google Scholar] [CrossRef]
- Sierra-Mondragon, E.; Molina-Jijon, E.; Namorado-Tonix, C.; Rodriguez-Munoz, R.; Pedraza-Chaverri, J.; Reyes, J.L. Data on nephroprotective effect of all-trans retinoic acid in early diabetic nephropathy. Data Brief 2018, 20, 784–789. [Google Scholar] [CrossRef]
- Basu, T.K.; Basualdo, C. Vitamin A homeostasis and diabetes mellitus. Nutrition 1997, 13, 804–806. [Google Scholar] [CrossRef]
- Starkey, J.M.; Zhao, Y.; Sadygov, R.G.; Haidacher, S.J.; Lejeune, W.S.; Dey, N.; Luxon, B.A.; Kane, M.A.; Napoli, J.L.; Denner, L.; et al. Altered retinoic acid metabolism in diabetic mouse kidney identified by O isotopic labeling and 2D mass spectrometry. PloS ONE 2010, 5, e11095. [Google Scholar] [CrossRef] [PubMed]
- Guleria, R.S.; Singh, A.B.; Nizamutdinova, I.T.; Souslova, T.; Mohammad, A.A.; Kendall, J.A., Jr.; Baker, K.M.; Pan, J. Activation of retinoid receptor-mediated signaling ameliorates diabetes-induced cardiac dysfunction in Zucker diabetic rats. J. Mol. Cell. Cardiol. 2013, 57, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arreola-Mendoza, L.; Reyes, J.L.; Melendez, E.; Martin, D.; Namorado, M.C.; Sanchez, E.; Del Razo, L.M. Alpha-tocopherol protects against the renal damage caused by potassium dichromate. Toxicology 2006, 218, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Molina-Jijon, E.; Rodriguez-Munoz, R.; Namorado Mdel, C.; Pedraza-Chaverri, J.; Reyes, J.L. Oxidative stress induces claudin-2 nitration in experimental type 1 diabetic nephropathy. Free Radic. Biol. Med. 2014, 72, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Lane, P.H.; Steffes, M.W.; Mauer, S.M. Estimation of glomerular volume: a comparison of four methods. Kidney Int. 1992, 41, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zhang, L.; Yuan, X.; Hao, J.; Ni, J.; Hao, L. Upregulation of allograft inflammatory factor1 expression and secretion by macrophages stimulated with aldosterone promotes renal fibroblasts to a profibrotic phenotype. Int. J. Mol. Med. 2018, 42, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Tam, F.W.K.; Ong, A.C.M. Renal monocyte chemoattractant protein-1: an emerging universal biomarker and therapeutic target for kidney diseases? Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef]
- Srinivas, H.; Juroske, D.M.; Kalyankrishna, S.; Cody, D.D.; Price, R.E.; Xu, X.C.; Narayanan, R.; Weigel, N.L.; Kurie, J.M. c-Jun N-terminal kinase contributes to aberrant retinoid signaling in lung cancer cells by phosphorylating and inducing proteasomal degradation of retinoic acid receptor alpha. Mol. Cell. Biol. 2005, 25, 1054–1069. [Google Scholar] [CrossRef]
- Singh, A.B.; Guleria, R.S.; Nizamutdinova, I.T.; Baker, K.M.; Pan, J. High glucose-induced repression of RAR/RXR in cardiomyocytes is mediated through oxidative stress/JNK signaling. J. Cell. Physiol. 2012, 227, 2632–2644. [Google Scholar] [CrossRef]
- Parving, H.H.; Persson, F.; Rossing, P. Microalbuminuria: a parameter that has changed diabetes care. Diabetes Res. Clin. Pract. 2015, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Shehata, N.I.; ElNokeety, M.M.; El-Emady, Y.F. Potential serum biomarkers for early detection of diabetic nephropathy. Diabetes Res. Clin. Pract. 2018, 136, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.H.; Roumelioti, M.E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, H.; Chen, H.; Zhang, M.; Ma, Q. High expression of neutrophil gelatinase-associated lipocalin (NGAL) in the kidney proximal tubules of diabetic rats. Adv. Med. Sci. 2015, 60, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Dechow, C.; Morath, C.; Lehrke, I.; Amann, K.; Waldherr, R.; Floege, J.; Ritz, E. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J. Am. Soc. Nephrol. 2000, 11, 1479–1487. [Google Scholar] [PubMed]
- Wang, Y.Y.; Jiang, H.; Pan, J.; Huang, X.R.; Wang, Y.C.; Huang, H.F.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y.; Chen, J.H. Macrophage-to-Myofibroblast Transition Contributes to Interstitial Fibrosis in Chronic Renal Allograft Injury. J. Am. Soc. Nephrol. 2017, 28, 2053–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Hatta, Y.; Iriyama, N.; Hasegawa, Y.; Uchida, H.; Nakagawa, M.; Makishima, M.; Takeuchi, J.; Takei, M. Induced differentiation of human myeloid leukemia cells into M2 macrophages by combined treatment with retinoic acid and 1alpha,25-dihydroxyvitamin D3. PloS ONE 2014, 9, e113722. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Skrypnyk, N.I.; Skvarca, L.B.; Penchev, R.; Zhang, K.X.; Rochon, E.R.; Fall, J.L.; Paueksakon, P.; Yang, H.; Alford, C.E.; et al. Retinoic Acid Signaling Coordinates Macrophage-Dependent Injury and Repair after AKI. J. Am. Soc. Nephrol. 2016, 27, 495–508. [Google Scholar] [CrossRef]
- Vellozo, N.S.; Pereira-Marques, S.T.; Cabral-Piccin, M.P.; Filardy, A.A.; Ribeiro-Gomes, F.L.; Rigoni, T.S.; DosReis, G.A.; Lopes, M.F. All-Trans Retinoic Acid Promotes an M1- to M2-Phenotype Shift and Inhibits Macrophage-Mediated Immunity to Leishmania major. Front. Immunol. 2017, 8, 1560. [Google Scholar] [CrossRef]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef]
- Wang, H.; Dan, Z.; Jiang, H. Effect of all-trans retinoic acid on liver fibrosis induced by common bile duct ligation in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 553–557. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Mennone, A.; Boyer, J.L.; Cai, S.Y. Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology 2011, 53, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.P.; Genta, S.; Oliveros, L.; Anzulovich, A.; Gimenez, M.S.; Sanchez, S.S. Vitamin A deficiency injures liver parenchyma and alters the expression of hepatic extracellular matrix. J. Appl. Toxicol. 2009, 29, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Guleria, R.S.; Singh, A.B.; Kendall, J.A., Jr.; Baker, K.M.; Pan, J. Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-kappaB signaling pathway. J. Cell. Physiol. 2013, 228, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Guleria, R.S.; Zhu, S.; Baker, K.M. Molecular Mechanisms of Retinoid Receptors in Diabetes-Induced Cardiac Remodeling. J. Clin. Med. 2014, 3, 566–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Mondragon, E.; Rodríguez-Muñoz, R.; Namorado-Tonix, C.; Molina-Jijon, E.; Romero-Trejo, D.; Pedraza-Chaverri, J.; Reyes, J.L. All-Trans Retinoic Acid Attenuates Fibrotic Processes by Downregulating TGF-β1/Smad3 in Early Diabetic Nephropathy. Biomolecules 2019, 9, 525. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100525
Sierra-Mondragon E, Rodríguez-Muñoz R, Namorado-Tonix C, Molina-Jijon E, Romero-Trejo D, Pedraza-Chaverri J, Reyes JL. All-Trans Retinoic Acid Attenuates Fibrotic Processes by Downregulating TGF-β1/Smad3 in Early Diabetic Nephropathy. Biomolecules. 2019; 9(10):525. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100525
Chicago/Turabian StyleSierra-Mondragon, Edith, Rafael Rodríguez-Muñoz, Carmen Namorado-Tonix, Eduardo Molina-Jijon, Daniel Romero-Trejo, Jose Pedraza-Chaverri, and Jose L. Reyes. 2019. "All-Trans Retinoic Acid Attenuates Fibrotic Processes by Downregulating TGF-β1/Smad3 in Early Diabetic Nephropathy" Biomolecules 9, no. 10: 525. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100525