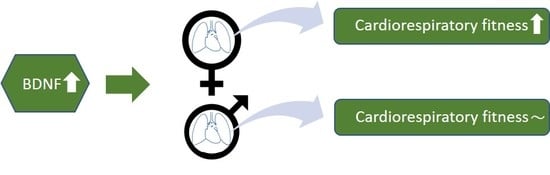
Sex-Specific Associations of Brain-Derived Neurotrophic Factor and Cardiorespiratory Fitness in the General Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Interview, Medical, and Laboratory Examination
2.3. Exercise Testing
2.4. Gas Exchange Variables
2.5. Statistics
3. Results
3.1. General Characteristics
3.2. The Association between BDNF and CRF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, T.; Fujimura, H.; Altar, C.A.; Li, J.; Kambayashi, J.; Tandon, N.N.; Sun, B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000, 470, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Donovan, M.J.; Miranda, R.C.; Kraemer, R.; McCaffrey, T.A.; Tessarollo, L.; Mahadeo, D.; Sharif, S.; Kaplan, D.R.; Tsoulfas, P.; Parada, L.; et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am. J. Pathol. 1995, 147, 309–324. [Google Scholar] [PubMed]
- Yamamoto, H.; Gurney, M.E. Human platelets contain brain-derived neurotrophic factor. J. Neurosci.: Off. J. Soc. Neurosci. 1990, 10, 3469–3478. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Kaess, B.M.; Preis, S.R.; Lieb, W.; Beiser, A.S.; Yang, Q.; Chen, T.C.; Hengstenberg, C.; Erdmann, J.; Schunkert, H.; Seshadri, S.; et al. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J. Am. Heart Assoc. 2015, 4, e001544. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, Y.; Zhang, Y.; Chen, Z.Y. Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochem. Biophys. Res. Commun. 2011, 415, 99–103. [Google Scholar] [CrossRef]
- Takashio, S.; Sugiyama, S.; Yamamuro, M.; Takahama, H.; Hayashi, T.; Sugano, Y.; Izumiya, Y.; Hokimoto, S.; Minamino, N.; Yasuda, S.; et al. Significance of low plasma levels of brain-derived neurotrophic factor in patients with heart failure. Am. J. Cardiol. 2015, 116, 243–249. [Google Scholar] [CrossRef]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report, 2018. Washington, DC; US. Department of Health and Human Services. 2018. Available online: https://health.gov/paguidelines/second-edition/report/pdf/pag_advisory_committee_report.pdf (accessed on 1 April 2019).
- Kokkinos, P.F.; Faselis, C.; Myers, J.; Narayan, P.; Sui, X.; Zhang, J.; Lavie, C.J.; Moore, H.; Karasik, P.; Fletcher, R. Cardiorespiratory Fitness and Incidence of Major Adverse Cardiovascular Events in US Veterans: A Cohort Study. Mayo. Clin. Proc. 2017, 92, 39–48. [Google Scholar] [CrossRef]
- Matthews, V.B.; Astrom, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerstrom, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscheweyh, R.; Willemer, C.; Kruger, K.; Duning, T.; Warnecke, T.; Sommer, J.; Volker, K.; Ho, H.V.; Mooren, F.; Knecht, S.; et al. Physical activity and memory functions: An interventional study. Neurobiol. Aging 2011, 32, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Rojas Vega, S.; Struder, H.K.; Vera Wahrmann, B.; Schmidt, A.; Bloch, W.; Hollmann, W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006, 1121, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.W.; Chu, E.; Hui, T.; Helmeste, D.; Law, C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci. Lett. 2008, 431, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Currie, J.; Ramsbottom, R.; Ludlow, H.; Nevill, A.; Gilder, M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci. Lett. 2009, 451, 152–155. [Google Scholar] [CrossRef]
- Nofuji, Y.; Suwa, M.; Moriyama, Y.; Nakano, H.; Ichimiya, A.; Nishichi, R.; Sasaki, H.; Radak, Z.; Kumagai, S. Decreased serum brain-derived neurotrophic factor in trained men. Neurosci. Lett. 2008, 437, 29–32. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, J.; Davis, J.M.; Blair, S.N.; Cho, H.C. Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy Korean men. Eur. J. Appl. Physiol. 2011, 111, 303–311. [Google Scholar] [CrossRef]
- Cho, H.C.; Kim, J.; Kim, S.; Son, Y.H.; Lee, N.; Jung, S.H. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO(2)max performance in healthy college men. Neurosci. Lett. 2012, 519, 78–83. [Google Scholar] [CrossRef]
- Jehn, C.F.; Becker, B.; Flath, B.; Nogai, H.; Vuong, L.; Schmid, P.; Luftner, D. Neurocognitive function, brain-derived neurotrophic factor (BDNF) and IL-6 levels in cancer patients with depression. J. Neuroimmunol. 2015, 287, 88–92. [Google Scholar] [CrossRef]
- Papasavvas, T.; Bonow, R.O.; Alhashemi, M.; Micklewright, D. Depression Symptom Severity and Cardiorespiratory Fitness in Healthy and Depressed Adults: A Systematic Review and Meta-Analysis. Sports Med. (Auckl. N.Z.) 2016, 46, 219–230. [Google Scholar] [CrossRef]
- Chan, K.L.; Tong, K.Y.; Yip, S.P. Relationship of serum brain-derived neurotrophic factor (BDNF) and health-related lifestyle in healthy human subjects. Neurosci. Lett. 2008, 447, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Goltz, A.; Janowitz, D.; Hannemann, A.; Nauck, M.; Hoffmann, J.; Seyfart, T.; Völzke, H.; Terock, J.; Grabe, H.J. Association of Brain-Derived Neurotrophic Factor and Vitamin D with Depression and Obesity: A Population-Based Study. Neuropsychobiology 2017, 76, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1beta, BDNF and synaptic plasticity. Neuropharmacology 2015, 96, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.A.; Arena, R.; Ellingsen, O.; Harber, M.P.; Myers, J.; Ozemek, C.; Ross, R. Cardiorespiratory fitness and cardiovascular disease—The past, present, and future. Prog. Cardiovasc. Dis. 2019, 62, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.; Koch, B.; Ittermann, T.; Schaper, C.; Dorr, M.; Felix, S.B.; Volzke, H.; Ewert, R.; Hansen, J.E. Influence of age, sex, body size, smoking, and beta blockade on key gas exchange exercise parameters in an adult population. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 469–476. [Google Scholar] [PubMed]
- Delezie, J.; Weihrauch, M.; Maier, G.; Tejero, R.; Ham, D.J.; Gill, J.F.; Karrer-Cardel, B.; Ruegg, M.A.; Tabares, L.; Handschin, C. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 16111–16120. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Brobst, D.; Chan, W.S.; Tse, M.C.L.; Herlea-Pana, O.; Ahuja, P.; Bi, X.; Zaw, A.M.; Kwong, Z.S.W.; Jia, W.H.; et al. Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility. Sci. Signal. 2019, 12, eaau1468. [Google Scholar] [CrossRef]
- Volzke, H.; Ittermann, T.; Schmidt, C.O.; Baumeister, S.E.; Schipf, S.; Alte, D.; Biffar, R.; John, U.; Hoffmann, W. Prevalence trends in lifestyle-related risk factors. Dtsch. Arztebl. Int. 2015, 112, 185–192. [Google Scholar] [CrossRef]
- Völzke, H. Study of Health in Pomerania (SHIP). Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2012, 55, 790–794. [Google Scholar] [CrossRef]
- Volzke, H.; Haring, R.; Lorbeer, R.; Wallaschofski, H.; Reffelmann, T.; Empen, K.; Rettig, R.; John, U.; Felix, S.B.; Dorr, M. Heart valve sclerosis predicts all-cause and cardiovascular mortality. Atherosclerosis 2010, 209, 606–610. [Google Scholar] [CrossRef]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J. Am. Soc. Echocardiogr.: Off. Publ. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function--measured and estimated glomerular filtration rate. New Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.L.; Makrides, L.; Hitchcock, C.; Chypchar, T.; McCartney, N. Normal standards for an incremental progressive cycle ergometer test. Am. Rev. Respir. Dis. 1985, 131, 700–708. [Google Scholar] [PubMed]
- Koch, B.; Schaper, C.; Ittermann, T.; Spielhagen, T.; Dorr, M.; Volzke, H.; Opitz, C.F.; Ewert, R.; Glaser, S. Reference values for cardiopulmonary exercise testing in healthy volunteers: The SHIP study. Eur. Respir. J. 2009, 33, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Whipp, B.J.; Froelicher, V.F. Principles of Exercise Testing and Interpretation. J. Cardiopulm. Rehabil. Prev. 1987, 7, 189. [Google Scholar] [CrossRef]
- Chacon-Fernandez, P.; Sauberli, K.; Colzani, M.; Moreau, T.; Ghevaert, C.; Barde, Y.A. Brain-derived Neurotrophic Factor in Megakaryocytes. J. Biol. Chem. 2016, 291, 9872–9881. [Google Scholar] [CrossRef] [Green Version]
- Stone, C.J.; Koo, C.-Y. Additive splines in statistics. Proc. Stat. Comp. Sect. Am. Statist. Assoc. 1985, 27, 45–48. [Google Scholar]
- Zoladz, J.A.; Pilc, A.; Majerczak, J.; Grandys, M.; Zapart-Bukowska, J.; Duda, K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol.: Off. J. Pol. Physiol. Soc. 2008, 59, 119–132. [Google Scholar]
- Seifert, T.; Brassard, P.; Wissenberg, M.; Rasmussen, P.; Nordby, P.; Stallknecht, B.; Adser, H.; Jakobsen, A.H.; Pilegaard, H.; Nielsen, H.B.; et al. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R372–R377. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Moller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Swardfager, W.; Herrmann, N.; Marzolini, S.; Saleem, M.; Shammi, P.; Oh, P.I.; Albert, P.R.; Daigle, M.; Kiss, A.; Lanctot, K.L. Brain derived neurotrophic factor, cardiopulmonary fitness and cognition in patients with coronary artery disease. Brain Behav. Immun. 2011, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejri, I.; Grimm, A.; Eckert, A. Mitochondria, Estrogen and Female Brain Aging. Front. Aging Neurosci. 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundsgaard, A.M.; Kiens, B. Gender differences in skeletal muscle substrate metabolism-molecular mechanisms and insulin sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J. Age dependency of oxygen uptake and related parameters in exercise testing: An expert opinion on reference values suitable for adults. Lung 2013, 191, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.M.; Lavin, K.M.; Many, G.M.; Thalacker-Mercer, A.; Merritt, E.K.; Bickel, C.S.; Mayhew, D.L.; Tuggle, S.C.; Cross, J.M.; Kosek, D.J.; et al. Human neuromuscular aging: Sex differences revealed at the myocellular level. Exp. Gerontol. 2018, 106, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Kinugawa, S.; Matsushima, S.; Mizushima, W.; Fukushima, A.; Furihata, T.; Kadoguchi, T.; Yokota, T.; Suga, T.; Okita, K.; et al. Brain-Derived Neurotrophic Factor Maintains Exercise Capacity and Mitochondrial Function in the Skeletal Muscle Through Ampk-Pgc1α Signaling. Circulation 2014, 130, A12182. [Google Scholar]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J. Cachexiasarcopenia Muscle 2016, 7, 290–298. [Google Scholar] [CrossRef]
- Tay, L.; Ding, Y.Y.; Leung, B.P.; Ismail, N.H.; Yeo, A.; Yew, S.; Tay, K.S.; Tan, C.H.; Chong, M.S. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age 2015, 37, 121. [Google Scholar] [CrossRef]
- Foltran, R.B.; Diaz, S.L. BDNF isoforms: A round trip ticket between neurogenesis and serotonin? J. Neurochem. 2016, 138, 204–221. [Google Scholar] [CrossRef]


| VO2peak Quartiles | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| mL/min | 1667–2155 | 2104–2606 | 2409.5–2953 | 2822–3454 | p for Trend | |
| n | 191 | 199 | 196 | 199 | ||
| Age (years) | 49 (39; 59) | 49 (39; 59) | 49 (39; 58) | 49 (38; 59) | 0.9167 | |
| BDNF (ng/mL) | 21.76 (17.33; 25.86) | 21.73 (17.23; 26.77) | 20.87 (17.34; 24.58) | 21.50 (17.96; 25.63) | 0.4102 | |
| Risk factors | BMI (kg/m2) | 27.0 (24.4; 29.5) | 27.1 (25.0; 29.6) | 27.6 (25.5; 30.7) | 27.9 (25.4; 30.4) | 0.0236 |
| Hypertension (%) | 50 | 47.47 | 50.77 | 41.21 | 0.2155 | |
| Systolic BP (mmHg) | 132 (120; 143) | 132 (125; 142) | 133 (123; 142) | 132 (122; 142) | 0.8439 | |
| Diabetes mellitus (%) | 7.9 | 7.5 | 7.7 | 4 | 0.3654 | |
| LVEF (%) | 69 (63; 77) | 71 (66; 77) | 72 (65; 79) | 71 (65; 77) | 0.2617 | |
| Smoking (%) | 42.4 | 25.3 | 22 | 10.6 | <0.0001 | |
| Physical inactivity (%) | 31.4 | 19.1 | 16.8 | 10 | <0.0001 | |
| eGFR (mL/min/1.72 mm2) | 103 (89; 115) | 106 (96; 115) | 106 (97; 114) | 106 (95; 113) | 0.3049 | |
| BIA | Fat mass (%) | 23.2 (19.2; 26.8) | 23.65 (19.6; 26.5) | 23.7 (19.6; 27) | 22.45 (19; 25.4) | 0.1457 |
| Lean mass (kg) | 63.3 (57.9; 68.3) | 64.7 (60; 69.9) | 67.4 (62.8; 72.7) | 69.65 (64.9; 74.4) | <0.0001 | |
| ECM (kg) | 29.2 (26.3; 31.9) | 28.9 (27.2; 31.6) | 30.1 (27.7; 33.2) | 30.9 (28.5; 34.0) | <0.0001 | |
| BCM (kg) | 34.3 (30.2; 37.6) | 35.2 (32.8; 38.6) | 37.0 (34.3; 40.0) | 38.6 (35.2; 41.6) | <0.0001 | |
| BW (L) | 46.4 (42.4; 50.0) | 47.4 (43.9; 51.2) | 49.4 (46.1; 53.2) | 50.9 (47.5; 54.5) | <0.0001 | |
| Lipids | Total cholesterol (mmol/L) | 5.5 (4.7; 6.4) | 5.3 (4.6; 6.1) | 5.4 (4.7; 6.2) | 5.3 (4.5; 6) | 0.1398 |
| TG (mmol/L) | 1.4 (1.01; 2.37) | 1.35 (0.91; 1.98) | 1.43 (0.94; 2.16) | 1.25 (0.9; 1.89) | 0.0573 | |
| LDLC (mmol/L) | 3.58 (2.89; 4.19) | 3.4 (2.78; 3.98) | 3.44 (2.89; 4.0) | 3.34 (2.68; 3.92) | 0.1653 | |
| HDL Chol (mmol/L) | 1.22 (1.02; 1.44) | 1.3 (1.11; 1.53) | 1.25 (1.09; 1.47) | 1.33 (1.14; 1.55) | 0.0016 | |
| Depression | Feelings of sadness/depressed mood for a period of at least 2 weeks (%) | 39.27 | 32.83 | 29.08 | 34.67 | 0.2003 |
| Lack of interest, tiredness, or loss of energy for a period of at least 2 weeks (%) | 23.04 | 20.71 | 11.22 | 9.55 | 0.0002 | |
| CPET | VO2 peak (mL/min/kg) | 22.4 (19.0; 26.6) | 27.8 (24.6; 31.6) | 29.9 (26.2; 35.7) | 34.5 (30.3; 41.2) | <0.0001 |
| VO2AT (mL/min) | 900 (850; 1050) | 1100 (950; 1250) | 1200 (1100; 1350) | 1400 (1250; 1550) | <0.0001 | |
| Watt max | 164 (132; 180) | 196 (164; 228) | 212 (180; 244) | 244 (212; 276) | <0.0001 | |
| HR max (/min) | 153 (137; 171) | 166 (148; 181) | 166 (148; 179) | 171 (162; 181) | <0.0001 | |
| VO2peak Quartiles | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| mL/min | 1118.5–1370 | 1391–1650 | 1566–1900 | 1885–2200 | p for Trend | |
| n | 204 | 200 | 204 | 214 | ||
| Age (years) | 48 (38; 59) | 47 (38; 58) | 48 (38; 59) | 47 (39; 60) | 0.9883 | |
| BDNF (ng/mL) | 22.09 (18.58; 26.30) | 22.65 (18.34; 25.87) | 22.3 (18.68; 26.88) | 23.34 (19.38; 27.23) | 0.1427 | |
| Risk factors | BMI (kg/m2) | 24.4 (21.8; 28.0) | 25.5 (23.2; 27.9) | 26.3 (23.4; 30.1) | 26.9 (24.0; 30.5) | <0.0001 |
| Hypertension (%) | 31.4 | 27.6 | 31.9 | 31.3 | 0.7811 | |
| Systolic BP (mmHg) | 116 (107; 129) | 117 (108; 130) | 116 (106; 127) | 118 (111; 128) | 0.1902 | |
| Diabetes mellitus (%) | 4.9 | 8 | 7.4 | 3.7 | 0.2139 | |
| LVEF (%) | 72 (67; 77) | 73 (67; 79) | 73 (67; 78) | 75 (67; 80) | 0.5086 | |
| Smoking (%) | 31.4 | 24.5 | 17.7 | 18.2 | 0.0211 | |
| Physical inactivity (%) | 25 | 16.5 | 13.7 | 7.5 | <0.0001 | |
| eGFR (mL/min/1.72 mm2) | 106 (92; 116) | 105 (93; 114) | 104 (91; 113) | 104 (93; 114) | 0.2082 | |
| BIA | Fat mass (kg) | 31.75 (26.8; 36.6) | 32.6 (28.2; 36.4) | 33.75 (28.75; 38.65) | 34.4 (28.9; 38.2) | 0.0084 |
| Lean mass (kg) | 44.45 (41.2; 47.8) | 46.4 (43.6; 48.9) | 47.3 (44.4; 51.6) | 49.4 (46.7; 52.4) | <0.0001 | |
| ECM (kg) | 21.9 (20.1; 23.5) | 22.5 (20.9; 24.3) | 23.1 (21.5; 25.1) | 23.5 (22.0; 25.5) | < 0,0001 | |
| BCM (kg) | 22.5 (20.7; 24.6) | 23.6 (21.9; 25.2) | 24.6 (22.5; 26.8) | 25.7 (24.0; 27.9) | <0.0001 | |
| BW (L) | 32.5 (30.2; 35.0) | 34.0 (32.0; 35.8) | 34.7 (32.6; 37.8) | 36.1 (34.2; 38.4) | <0.0001 | |
| Lipids | Total cholesterol (mmol/L) | 5.5 (4.9; 6.3) | 5.5 (4.8; 6.2) | 5.2 (4.6; 6.0) | 5.5 (4.8; 6.3) | 0.0467 |
| TG (mmol/L) | 1.2 (0.9; 1.8) | 1.1 (0.8; 1.6) | 1.2 (0.9; 1.6) | 1.1 (0.8; 1.5) | 0.1023 | |
| LDLC (mmol/L) | 3.3 (2.8; 4.0) | 3.3 (2.7; 4.1) | 3.2 (2.6; 3.8) | 3.3 (2.7; 3.9) | 0.6075 | |
| HDL Chol (mmol/L) | 1.6 (1.4; 1.9) | 1.6 (1.4; 1.9) | 1.6 (1.3; 1.8) | 1.6 (1.4; 1.8) | 0.2558 | |
| Depression | Feelings of sadness/depressed mood for a period of at least 2 weeks (%) | 53.43 | 54.5 | 51.93 | 49.07 | 0.7066 |
| Lack of interest, tiredness, or loss of energy for a period of at least 2 weeks (%) | 39.22 | 37.5 | 38.73 | 32.71 | 0.4934 | |
| CPET | VO2 peak (mL/min/kg) | 18.6 (16.0; 22.0) | 22.4 (19.4; 25.2) | 23.7 (19.8; 28.3) | 26.9 (23.1; 31.8) | <0.0001 |
| VO2AT (mL/min) | 700 (650; 800) | 800 (750; 900) | 900 (800; 975) | 1050 (900; 1150) | <0.0001 | |
| Watt max | 116 (84; 132) | 132 (116; 148) | 148 (116; 148) | 164 (148; 180) | <0.0001 | |
| HR max (/min) | 156 (134; 169) | 160 (142; 173) | 164 (148; 173) | 166 (151; 176) | <0.0001 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmalhofer, M.-L.; Markus, M.R.P.; Gras, J.C.; Kopp, J.; Janowitz, D.; Grabe, H.-J.; Groß, S.; Ewert, R.; Gläser, S.; Albrecht, D.; et al. Sex-Specific Associations of Brain-Derived Neurotrophic Factor and Cardiorespiratory Fitness in the General Population. Biomolecules 2019, 9, 630. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100630
Schmalhofer M-L, Markus MRP, Gras JC, Kopp J, Janowitz D, Grabe H-J, Groß S, Ewert R, Gläser S, Albrecht D, et al. Sex-Specific Associations of Brain-Derived Neurotrophic Factor and Cardiorespiratory Fitness in the General Population. Biomolecules. 2019; 9(10):630. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100630
Chicago/Turabian StyleSchmalhofer, Marie-Lena, Marcello R.P. Markus, Jan C. Gras, Juliane Kopp, Deborah Janowitz, Hans-Jörgen Grabe, Stefan Groß, Ralf Ewert, Sven Gläser, Diana Albrecht, and et al. 2019. "Sex-Specific Associations of Brain-Derived Neurotrophic Factor and Cardiorespiratory Fitness in the General Population" Biomolecules 9, no. 10: 630. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100630