Protective Effect of Panaxynol Isolated from Panax vietnamensis against Cisplatin-Induced Renal Damage: In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. HPLC Analysis of Panaxynol
2.3. Extraction and Isolation
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Quantification of Apoptosis with Image-Based Cytometric Assay
2.7. Western Blot Analysis
2.8. Cisplatin-Induced Nephrotoxicity in Mice
2.9. Determination of Serum Creatinine and Blood Urea Nitrogen
2.10. Real-Time PCR for Cyclooxygenase-2, Monocyte Chemoattractant Protein-1, and Hypoxanthine Phosphoribosyltransferase-1
2.11. Statistical Analysis
3. Results
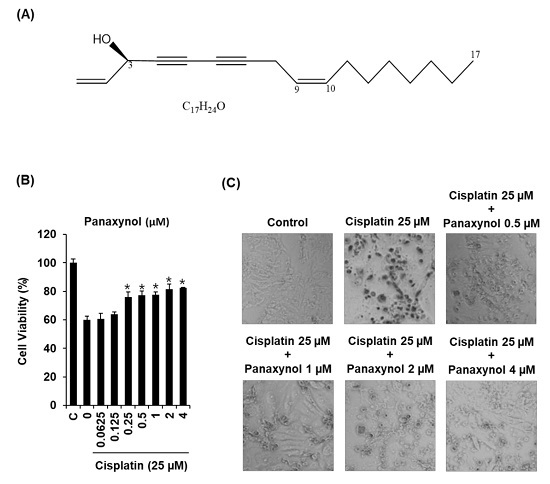
3.1. Protective Effect of Panaxynol Isolated from Panax vietnamensis on Cisplatin-Induced LLC-PK1 Cell Death
3.2. Effect of Panaxynol Isolated from Panax vietnamensis on Cisplatin-Induced Apoptosis in LLC-PK1 Cells
3.3. Effect of Panaxynol Isolated from Panax vietnamensis on Expression Levels of JNK, P38, and Cleaved Caspase-3 in Cisplatin-Treated LLC-PK1 Cells
3.4. Effects of Panaxynol Isolated from Panax vietnamensis on Cisplatin-Induced Nephrotoxicity in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Firsov, D.; Tokonami, N.; Bonny, O. Role of the renal circadian timing system in maintaining water and electrolytes homeostasis. Mol. Cell Endocrinol. 2012, 349, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Wensing, K.U.; Ciarimboli, G. Saving ears and kidneys from cisplatin. Anticancer Res. 2013, 33, 4183–4188. [Google Scholar] [PubMed]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press. 2014, 12, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari-Soreshjani, S.; Asadi-Samani, M.; Yang, Q.; Saeedi-Boroujeni, A. Phytotherapy of nephrotoxicity-induced by cancer drugs: An updated review. J. Nephropathol. 2017, 6, 254–263. [Google Scholar] [CrossRef]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Renal. Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef]
- Xing, J.J.; Hou, J.G.; Ma, Z.N.; Wang, Z.; Ren, S.; Wang, Y.P.; Liu, W.C.; Chen, C.; Li, W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019, e12627. [Google Scholar] [CrossRef]
- Qi, Z.; Li, W.; Tan, J.; Wang, C.; Lin, H.; Zhou, B.; Liu, J.; Li, P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine 2019, 61, 152862. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.-F.; Han, X.-Y.; Sun, Y.-S.; Zhang, L.-X.; Liu, W.; Liu, X.-X.; Li, W.; Liu, Y.-Y. Kidney protection effect of ginsenoside re and its underlying mechanisms on cisplatin-induced kidney injury. Cell Physiol. Biochem. 2018, 48, 2219–2229. [Google Scholar] [CrossRef]
- Li, W.; Yan, M.-H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.-S. Ginsenoside Rg5 ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress, and apoptosis. Nutrients 2016, 8, 566. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.-H.; Shin, B.-K.; Kim, N.J.; Chang, S.-Y.; Park, J.H. Protective effect of ginsenosides Rk3 and Rh4 on cisplatin-induced acute kidney injury in vitro and in vivo. J. Ginseng Res. 2017, 41, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokozawa, T.; Wu Liu, Z. The role of ginsenoside-Rd in cisplatin-induced acute renal failure. Ren. Fail. 2000, 22, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Dong, E. Role of ginsenoside-Rd in cisplatin-induced renal injury: Special reference to DNA fragmentation. Nephron 2001, 89, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Kang, K.S. Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J. Ginseng Res. 2017, 41, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-S.; Han, I.-H.; Lee, D.; An, J.M.; Kim, S.-N.; Shin, M.-S.; Yamabe, N.; Hwang, G.S.; Yoo, H.H.; Choi, S.-J. Beneficial effects of fermented black ginseng and its ginsenoside 20 (S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J. Ginseng Res. 2016, 40, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- P Christensen, L. Aliphatic C17-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef]
- Knispel, N.; Ostrozhenkova, E.; Schramek, N.; Huber, C.; Pena-Rodriguez, L.M.; Bonfill, M.; Palazon, J.; Wischmann, G.; Cusido, R.M.; Eisenreich, W. Biosynthesis of panaxynol and panaxydol in Panax ginseng. Molecules 2013, 18, 7686–7698. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.L.; Han, T.; Wu, J.Z.; Zhang, Q.Y.; Zhang, H.; Huang, B.K.; Rahman, K.; Qin, L.P. Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of Panax ginseng and its endophytic fungus. Phytomedicine 2009, 16, 609–616. [Google Scholar] [CrossRef]
- Le, H.T.; Nguyen, H.T.; Min, H.Y.; Hyun, S.Y.; Kwon, S.; Lee, Y.; Le, T.H.V.; Lee, J.; Park, J.H.; Lee, H.Y. Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells. Cancer Lett. 2018, 412, 297–307. [Google Scholar] [CrossRef]
- Rahman, M.A.; Cho, S.C.; Song, J.; Mun, H.T.; Moon, S.S. Dendrazawaynes A and B, antifungal polyacetylenes from Dendranthema zawadskii (Asteraceae). Planta Med. 2007, 73, 1089–1094. [Google Scholar] [CrossRef]
- Qu, C.; Li, B.; Lai, Y.; Li, H.; Windust, A.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.; Wang, X.L.; Tang, D. Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy. J. Ethnopharmacol. 2015, 168, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparala, A.; Poudyal, D.; Witalison, E.E.; Tashkandi, H.; Chumanevich, A.A.; Hofseth, J.L.; Pittman, D.L.; Wyatt, M.D.; Windust, A.; Nagarkatti, M. Panaxynol, a bioactive component of American ginseng, targets macrophages and suppresses colitis in mice. Preprints 2018, 2018030146. [Google Scholar] [CrossRef]
- Nie, B.-M.; Jiang, X.-Y.; Cai, J.-X.; Fu, S.-L.; Yang, L.-M.; Lin, L.; Hang, Q.; Lu, P.-L.; Lu, Y. Panaxydol and panaxynol protect cultured cortical neurons against Aβ25–35-induced toxicity. Neuropharmacology 2008, 54, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Kimura, K.; Nakashima, K.-i.; Inoue, M. Ameliorative effect of panaxynol on the reduction in high-molecular-weight adiponectin secretion from 3T3-L1 adipocytes treated with palmitic acids. Eur. J. Pharmacol. 2018, 820, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influence of processing and storage on its content in carrots (Daucus carota L.). J. Sci. Food Agr. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Peres, L.A.B.; Cunha Júnior, A.D.D. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Hajian, S.; Rafieian-Kopaei, M.; Nasri, H. Renoprotective effects of antioxidants against cisplatin nephrotoxicity. J. Nephropharmacol. 2014, 3, 39. [Google Scholar]
- Ridzuan, N.R.; Rashid, N.A.; Othman, F.; Budin, S.B.; Hussan, F.; Teoh, S.L. Protective role of natural products in cisplatin-induced nephrotoxicity. Mini Rev. Med. Chem. 2019, 19, 1134–1143. [Google Scholar] [CrossRef]
- Ojha, S.; Venkataraman, B.; Kurdi, A.; Mahgoub, E.; Sadek, B.; Rajesh, M. Plant-derived agents for counteracting cisplatin-induced nephrotoxicity. Oxid Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Yoshikura, M. Studies on the Components of Panax Ginseng C. A. Meyer. 3. on the Ethereal Extract of Ginseng Radix Alba. (3). on the Structure of a new Acetylene Derivative “panaxynol”. Yakugaku Zasshi 1964, 84, 757–759. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, A.M.; Al Salam, S.; AlMahruqi, A.S.; Al Husseni, I.S.; Mansour, M.A.; Ali, B.H. N-acetylcysteine improves renal hemodynamics in rats with cisplatin-induced nephrotoxicity. J. Appl Toxicol. 2010, 30, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Feinfeld, D.A. N-acetylcysteine as salvage therapy in cisplatin nephrotoxicity. Ren. Fail. 2002, 24, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Shalby, A.B.; Assaf, N.; Ahmed, H.H. Possible mechanisms for N-acetyl cysteine and taurine in ameliorating acute renal failure induced by cisplatin in rats. Toxicol. Mech. Methods 2011, 21, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, M.E.; Quiroga, A.G.; Castro, J.; Ortiz, A.; Aller, P.; Mata, F. Inhibition of p38-MAPK potentiates cisplatin-induced apoptosis via GSH depletion and increases intracellular drug accumulation in growth-arrested kidney tubular epithelial cells. Toxicol. Sci. 2009, 111, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Kim, K.H.; Lee, W.Y.; Kim, C.-E.; Sung, S.H.; Kang, K.B.; Kang, K.S. Multiple targets of 3-dehydroxyceanothetric acid 2-methyl ester to protect against cisplatin-induced cytotoxicity in kidney epithelial LLC-PK1 cells. Molecules 2019, 24, 878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Choi, P.; Kim, T.; Ko, H.; Kim, H.K.; Kang, K.S.; Ham, J. Protective effects of processed ginseng and its active ginsenosides on cisplatin-induced nephrotoxicity: In vitro and in vivo studies. J. Agric. Food Chem. 2015, 63, 5964–5969. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Večerić-Haler, Ž. Cisplatin-induced rodent model of kidney injury: Characteristics and challenges. BioMed Res. 2018, 2018, 1462802. [Google Scholar] [CrossRef]
- Sánchez-González, P.D.; López-Hernández, F.J.; López-Novoa, J.M.; Morales, A.I. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit. Rev. Toxicol. 2011, 41, 803–821. [Google Scholar] [CrossRef]
- Jung, K.; An, J.M.; Eom, D.W.; Kang, K.S.; Kim, S.N. Preventive effect of fermented black ginseng against cisplatin-induced nephrotoxicity in rats. J. Ginseng Res. 2017, 41, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jia, Z.; Sun, J.; Xu, L.; Zhao, B.; Yu, K.; Yang, M.; Yang, T.; Wang, R. Nitrooleic acid protects against cisplatin nephropathy: Role of COX-2/mPGES-1/PGE2 cascade. Mediators Inflamm. 2015, 2015. [Google Scholar] [CrossRef]
- Yu, X.; Meng, X.; Xu, M.; Zhang, X.; Zhang, Y.; Ding, G.; Huang, S.; Zhang, A.; Jia, Z. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-κB and improving mitochondrial function. EBioMedicine 2018, 36, 266–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Park, J.H.; Kim, H.S.; Lee, C.Y.; Lee, H.J.; Kang, K.S.; Kim, C.E. Systems-level mechanisms of action of Panax ginseng: A network pharmacological approach. J. Ginseng Res. 2018, 42, 98–106. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, J.; Vu-Huynh, K.L.; Van Le, T.H.; Tuoi Do, T.H.; Hwang, G.S.; Park, J.H.; Kang, K.S.; Nguyen, M.D.; Yamabe, N. Protective Effect of Panaxynol Isolated from Panax vietnamensis against Cisplatin-Induced Renal Damage: In Vitro and In Vivo Studies. Biomolecules 2019, 9, 890. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9120890
Lee D, Lee J, Vu-Huynh KL, Van Le TH, Tuoi Do TH, Hwang GS, Park JH, Kang KS, Nguyen MD, Yamabe N. Protective Effect of Panaxynol Isolated from Panax vietnamensis against Cisplatin-Induced Renal Damage: In Vitro and In Vivo Studies. Biomolecules. 2019; 9(12):890. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9120890
Chicago/Turabian StyleLee, Dahae, Jaemin Lee, Kim Long Vu-Huynh, Thi Hong Van Le, Thi Hong Tuoi Do, Gwi Seo Hwang, Jeong Hill Park, Ki Sung Kang, Minh Duc Nguyen, and Noriko Yamabe. 2019. "Protective Effect of Panaxynol Isolated from Panax vietnamensis against Cisplatin-Induced Renal Damage: In Vitro and In Vivo Studies" Biomolecules 9, no. 12: 890. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9120890