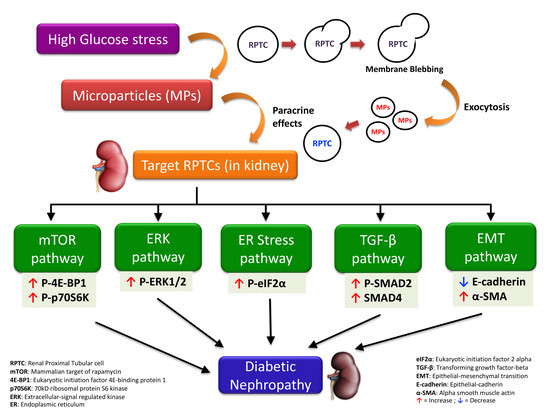
Microparticles as Potential Mediators of High Glucose-Induced Renal Cell Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation of Microparticles (MPs) from RPTCs
2.3. Treatment of Naïve RPTCs with MPs
2.4. Cell Viability Assay
2.5. Cell Cycle Analysis
2.6. Nuclear Extraction
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. High Glucose-Derived MPs Do Not Cause Cytotoxicity or Affect Cell Cycle in Naïve RPTCs
3.2. Continuous High Glucose-Derived MPs Activate mTOR Pathway in Naïve RPTCs
3.3. High Glucose-Derived MPs Activate ERK Pathway in Naïve RPTCs
3.4. High-Glucose Derived MPs Partially Activate ER Stress in Naïve RPTCs
3.5. Intermittent High Glucose-Derived MPs Activate the TGF-β Pathway in Naïve RPTCs
3.6. High Glucose-Derived MPs Induce EMT in Naïve RPTCs
4. Discussion
4.1. Cell Viability and Cell Cycle Progression
4.2. mTOR Pathway
4.3. ERK Pathway
4.4. ER Stress Response
4.5. TGF-β Pathway
4.6. EMT Pathway
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gheith, O.; Farouk, N.; Nampoory, N.; Halim, M.A.; Al-Otaibi, T. Diabetic kidney disease: World wide difference of prevalence and risk factors. J. Nephropharmacol. 2016, 5, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.O. The role of renal proximal tubular cells in diabetic nephropathy. Curr. Diabetes Rep. 2003, 3, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Gnudi, L.; Coward, R.J.M.; Long, D.A. Diabetic Nephropathy: Perspective on Novel Molecular Mechanisms. Trends Endocrinol. Metab. TEM 2016, 27, 820–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsaad, K.O.; Herzenberg, A.M. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: An update. J. Clin. Pathol. 2007, 60, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; Kuruvilla, V.; Wilbur, K.; Munusamy, S. Nephroprotective Effects of Metformin in Diabetic Nephropathy. J. Cell Physiol. 2017, 232, 731–742. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Morcos, M.; Sayed, A.A.; Bierhaus, A.; Yard, B.; Waldherr, R.; Merz, W.; Kloeting, I.; Schleicher, E.; Mentz, S.; Abd el Baki, R.F.; et al. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes 2002, 51, 3532–3544. [Google Scholar] [CrossRef]
- Zeni, L.; Norden, A.G.W.; Cancarini, G.; Unwin, R.J. A more tubulocentric view of diabetic kidney disease. J. Nephrol. 2017, 30, 701–717. [Google Scholar] [CrossRef]
- Mehta, R.L. Glycemic control and critical illness: Is the kidney involved? J. Am. Soc. Nephrol. 2007, 18, 2623–2627. [Google Scholar] [CrossRef]
- Brosius, F.C.; Khoury, C.C.; Buller, C.L.; Chen, S. Abnormalities in signaling pathways in diabetic nephropathy. Expert Rev. Endocrinol. Metab. 2010, 5, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.C.; Lai, K.N. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol. Dial. Transplant. 2012, 27, 3049–3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szablewski, L. Distribution of glucose transporters in renal diseases. J. Biomed. Sci. 2017, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Agouni, A.; Andriantsitohaina, R.; Martinez, M.C. Microparticles as biomarkers of vascular dysfunction in metabolic syndrome and its individual components. Curr. Vasc. Pharmacol. 2014, 12, 483–492. [Google Scholar] [CrossRef]
- Benameur, T.; Osman, A.; Parray, A.; Ait Hssain, A.; Munusamy, S.; Agouni, A. Molecular Mechanisms Underpinning Microparticle-Mediated Cellular Injury in Cardiovascular Complications Associated with Diabetes. Oxid. Med. Cell. Longev. 2019, 2019, 6475187. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Ma, K.L.; Ruan, X.Z.; Liu, B.C. The Emerging Roles of Microparticles in Diabetic Nephropathy. Int. J. Biol. Sci. 2017, 13, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Burger, D.; Turner, M.; Xiao, F.; Munkonda, M.N.; Akbari, S.; Burns, K.D. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia 2017, 60, 1791–1800. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, K.F.; Pietrani, N.T.; Fernandes, A.P.; Bosco, A.A.; de Sousa, M.C.R.; de Fatima Oliveira Silva, I.; Silveira, J.N.; Campos, F.M.F.; Gomes, K.B. Circulating microparticles levels are increased in patients with diabetic kidney disease: A case-control research. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 479, 48–55. [Google Scholar] [CrossRef]
- Burger, D.; Thibodeau, J.F.; Holterman, C.E.; Burns, K.D.; Touyz, R.M.; Kennedy, C.R. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J. Am. Soc. Nephrol. 2014, 25, 1401–1407. [Google Scholar] [CrossRef]
- Munkonda, M.N.; Akbari, S.; Landry, C.; Sun, S.; Xiao, F.; Turner, M.; Holterman, C.E.; Nasrallah, R.; Hebert, R.L.; Kennedy, C.R.J.; et al. Podocyte-derived microparticles promote proximal tubule fibrotic signaling via p38 MAPK and CD36. J. Extracell. Vesicles 2018, 7, 1432206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiong, M.; Fang, L.; Jiang, L.; Wen, P.; Dai, C.; Zhang, C.Y.; Yang, J. miR-21-containing microvesicles from injured tubular epithelial cells promote tubular phenotype transition by targeting PTEN protein. Am. J. Pathol. 2013, 183, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barteneva, N.S.; Fasler-Kan, E.; Bernimoulin, M.; Stern, J.N.; Ponomarev, E.D.; Duckett, L.; Vorobjev, I.A. Circulating microparticles: Square the circle. BMC Cell Biol. 2013, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Perez, J.C. The role of mTOR inhibitors in renal diseases. Nefrologia 2011, 31, 251–255. [Google Scholar] [PubMed]
- Peng, L.; Li, J.; Xu, Y.; Wang, Y.; Du, H.; Shao, J.; Liu, Z. The Protective Effect of Beraprost Sodium on Diabetic Nephropathy by Inhibiting Inflammation and p38 MAPK Signaling Pathway in High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Int. J. Endocrinol. 2016, 2016, 1690474. [Google Scholar] [CrossRef] [PubMed]
- Feliers, D.; Kasinath, B.S. Erk in kidney diseases. J. Signal Transduct. 2011, 2011, 768512. [Google Scholar] [CrossRef]
- Mariappan, M.M.; D’Silva, K.; Lee, M.J.; Sataranatarajan, K.; Barnes, J.L.; Choudhury, G.G.; Kasinath, B.S. Ribosomal biogenesis induction by high glucose requires activation of upstream binding factor in kidney glomerular epithelial cells. Am. J. Physiol. Renal. Physiol. 2011, 300, F219–F230. [Google Scholar] [CrossRef] [Green Version]
- Mason, R.M.; Wahab, N.A. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1358–1373. [Google Scholar] [CrossRef]
- Balasubramanyam, M.; Lenin, R.; Monickaraj, F. Endoplasmic reticulum stress in diabetes: New insights of clinical relevance. Indian J. Clin. Biochem. IJCB 2010, 25, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.K.; Hsu, S.P.; Wu, C.T.; Huang, J.W.; Cheng, H.T.; Chang, Y.W.; Hung, K.Y.; Wu, K.D.; Liu, S.H. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol. Med. 2011, 17, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Bottinger, E.P. TGF-beta in renal injury and disease. Semin. Nephrol. 2007, 27, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.H.; Kim, J.S.; Chang, J.W.; Kim, S.B.; Park, J.S.; Lee, S.K. AMP-activated protein kinase inhibits TGF-beta-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Am. J. Physiol. Renal. Physiol. 2013, 304, F686–F697. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Xiao, Y.; Shi, M.; Zhang, G.; Guo, B. SnoN as a key regulator of the high glucose-induced epithelial-mesenchymal transition in cells of the proximal tubule. Kidney Blood Press. Res. 2012, 35, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xue, H.; Yuan, P.; Ni, J.; Yu, C.; Huang, Y.; Lu, L.M. Angiotensin AT1 receptor activation mediates high glucose-induced epithelial-mesenchymal transition in renal proximal tubular cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, e152–e157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lian, X.; Liang, D.; Zhang, L.; Liu, S.; Yang, L.; Chi, Z.H.; Gu, H.F. Protective Effect of Znt7 on High Glucose-Induced Epithelial-to-Mesenchymal Transition in Renal Tubular Epithelial Cells. Kidney Blood Press. Res. 2018, 43, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Burns, W.C.; Twigg, S.M.; Forbes, J.M.; Pete, J.; Tikellis, C.; Thallas-Bonke, V.; Thomas, M.C.; Cooper, M.E.; Kantharidis, P. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: Implications for diabetic renal disease. J. Am. Soc. Nephrol. 2006, 17, 2484–2494. [Google Scholar] [CrossRef]
- Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 2004, 15, 1–12. [Google Scholar] [CrossRef]
- Agouni, A.; Mostefai, H.A.; Porro, C.; Carusio, N.; Favre, J.; Richard, V.; Henrion, D.; Martinez, M.C.; Andriantsitohaina, R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007, 21, 2735–2741. [Google Scholar] [CrossRef] [Green Version]
- Mostefai, H.A.; Agouni, A.; Carusio, N.; Mastronardi, M.L.; Heymes, C.; Henrion, D.; Andriantsitohaina, R.; Martinez, M.C. Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J. Immunol. 2008, 180, 5028–5035. [Google Scholar] [CrossRef]
- Pasha, M.; Sivaraman, S.K.; Frantz, R.; Agouni, A.; Munusamy, S. Metformin Induces Different Responses in Clear Cell Renal Cell Carcinoma Caki Cell Lines. Biomolecules 2019, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Allouch, S.; Munusamy, S. Metformin attenuates albumin-induced alterations in renal tubular cells in vitro. J. Cell. Physiol. 2017, 232, 3652–3663. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, W.; Levine, J.S. The role of the mammalian target of rapamycin (mTOR) in renal disease. J. Am. Soc. Nephrol. 2009, 20, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Kasinath, B.S.; Mariappan, M.M.; Sataranatarajan, K.; Lee, M.J.; Ghosh Choudhury, G.; Feliers, D. Novel mechanisms of protein synthesis in diabetic nephropathy--role of mRNA translation. Rev. Endocr. Metab. Disord. 2008, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Shechter, P.; Boner, G.; Rabkin, R. Tubular cell protein degradation in early diabetic renal hypertrophy. J. Am. Soc. Nephrol. 1994, 4, 1582–1587. [Google Scholar]
- Cunard, R. Endoplasmic Reticulum Stress in the Diabetic Kidney, the Good, the Bad and the Ugly. J. Clin. Med. 2015, 4, 715–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, M.; Yoshida, H. Endoplasmic reticulum stress in kidney function and disease. Curr. Opin. Nephrol. Hypertens 2015, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lee, K.; Wang, N.; He, J.C. The Role of Endoplasmic Reticulum Stress in Diabetic Nephropathy. Curr. Diabetes Rep. 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeyer, M.T.; Rastaldi, M.P.; Ikehata, M.; Neusser, M.A.; Kretzler, M.; Cohen, C.D.; Schlondorff, D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J. Am. Soc. Nephrol. 2008, 19, 2225–2236. [Google Scholar] [CrossRef]
- Ju, Y.; Su, Y.; Chen, Q.; Ma, K.; Ji, T.; Wang, Z.; Li, W.; Li, W. Protective effects of Astragaloside IV on endoplasmic reticulum stress-induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed. Pharmacother. 2019, 109, 84–92. [Google Scholar] [CrossRef]
- Safiedeen, Z.; Rodriguez-Gomez, I.; Vergori, L.; Soleti, R.; Vaithilingam, D.; Douma, I.; Agouni, A.; Leiber, D.; Dubois, S.; Simard, G.; et al. Temporal Cross Talk Between Endoplasmic Reticulum and Mitochondria Regulates Oxidative Stress and Mediates Microparticle-Induced Endothelial Dysfunction. Antioxid. Redox. Signal 2017, 26, 15–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoj Thomsen, L.; Fog-Tonnesen, M.; Nielsen Fink, L.; Norlin, J.; de Vinuesa, A.G.; Krarup Hansen, T.; de Heer, E.; Ten Dijke, P.; Rosendahl, A. Smad2 Phosphorylation in Diabetic Kidney Tubule Epithelial Cells Is Associated with Modulation of Several Transforming Growth Factor-beta Family Members. Nephron 2017, 135, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.T.; Melo, S.A.; Ozdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H., 2nd; LeBleu, V.S.; Kalluri, R. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gao, Y.; Xu, L.; Dang, W.; Yan, H.; Zou, D.; Zhu, Z.; Luo, L.; Tian, N.; Wang, X.; et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci. Rep. 2017, 7, 9371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Tang, L.Q.; Wei, W. Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFbeta1-PI3K/AKT pathway. Eur. J. Pharmacol. 2018, 824, 185–192. [Google Scholar] [CrossRef]
- Fragiadaki, M.; Mason, R.M. Epithelial-mesenchymal transition in renal fibrosis - evidence for and against. Int. J. Exp. Pathol. 2011, 92, 143–150. [Google Scholar] [CrossRef]
- Lu, Q.; Ji, X.J.; Zhou, Y.X.; Yao, X.Q.; Liu, Y.Q.; Zhang, F.; Yin, X.X. Quercetin inhibits the mTORC1/p70S6K signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in diabetic nephropathy. Pharmacol. Res. Off. J. Italian Pharmacol. Soc. 2015, 99, 237–247. [Google Scholar] [CrossRef]
- Dai, H.Y.; Zheng, M.; Tang, R.N.; Ni, J.; Ma, K.L.; Li, Q.; Liu, B.C. Effects of angiotensin receptor blocker on phenotypic alterations of podocytes in early diabetic nephropathy. Am. J. Med. Sci. 2011, 341, 207–214. [Google Scholar] [CrossRef]
- Liu, L.; Fu, W.; Xu, J.; Shao, L.; Wang, Y. Effect of BMP7 on podocyte transdifferentiation and Smad7 expression induced by hyperglycemia. Clin. Nephrol. 2015, 84, 95–99. [Google Scholar] [CrossRef]
- Wu, X.M.; Gao, Y.B.; Cui, F.Q.; Zhang, N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol. Open 2016, 5, 484–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omoto, S.; Nomura, S.; Shouzu, A.; Hayakawa, T.; Shimizu, H.; Miyake, Y.; Yonemoto, T.; Nishikawa, M.; Fukuhara, S.; Inada, M. Significance of platelet-derived microparticles and activated platelets in diabetic nephropathy. Nephron 1999, 81, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.Y.; Xu, R.J.; Zhang, S.H.; Qiao, Q.; Shen, L.; Li, M.; Xu, D.Y.; Wang, Z.Y. Alteration of circulatory platelet microparticles and endothelial microparticles in patients with chronic kidney disease. Int. J. Clin. Exp. Med. 2015, 8, 16704–16708. [Google Scholar] [PubMed]
- Lv, L.L.; Wu, W.J.; Feng, Y.; Li, Z.L.; Tang, T.T.; Liu, B.C. Therapeutic application of extracellular vesicles in kidney disease: Promises and challenges. J. Cell. Mol. Med. 2018, 22, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.; Aguera, M.L.; Luna-Ruiz, C.; Buendia, P.; Calleros, L.; Garcia-Jerez, A.; Rodriguez-Puyol, M.; Arias, M.; Arias-Guillen, M.; de Arriba, G.; et al. Markers of endothelial damage in patients with chronic kidney disease on hemodialysis. Am. J. Physiol. Renal. Physiol. 2017, 312, F673–F681. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravindran, S.; Pasha, M.; Agouni, A.; Munusamy, S. Microparticles as Potential Mediators of High Glucose-Induced Renal Cell Injury. Biomolecules 2019, 9, 348. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9080348
Ravindran S, Pasha M, Agouni A, Munusamy S. Microparticles as Potential Mediators of High Glucose-Induced Renal Cell Injury. Biomolecules. 2019; 9(8):348. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9080348
Chicago/Turabian StyleRavindran, Sreenithya, Mazhar Pasha, Abdelali Agouni, and Shankar Munusamy. 2019. "Microparticles as Potential Mediators of High Glucose-Induced Renal Cell Injury" Biomolecules 9, no. 8: 348. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9080348