Zebrafish Optomotor Response and Morphology Are Altered by Transient, Developmental Exposure to Bisphenol-A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
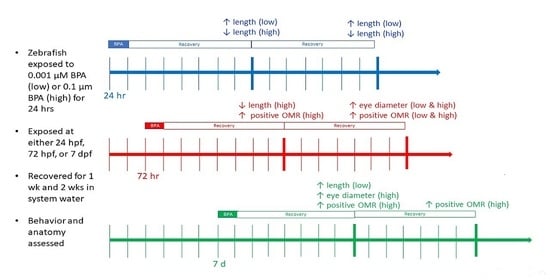
2.2. Exposures
2.3. Behavioral Assays
2.3.1. Startle Response
2.3.2. Optomotor Response
2.4. Morphometric Analysis
2.5. Statistical Analysis
3. Results
3.1. Suvival
3.2. Morphometric Results
3.2.1. 24 hpf Exposures
3.2.2. 72 hpf Exposures
3.3. Behavioral Responses
3.3.1. 24 hpf Exposures
3.3.2. 72 hpf Exposures
3.3.3. 7 dpf Exposures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-OH-A | 4-hydroxyandrostenedione |
| BPA | bisphenol-A |
| dpf | days post-fertilization |
| DMSO | dimethyl sulfoxide |
| EDCs | endocrine disrupting compounds |
| ER | estrogen receptor |
| ERRγ | estrogen related receptors |
| ED | eye diameter |
| GPER | G-protein coupled estrogen receptor |
| hpf | hours post-fertilization |
| NL | notochord length |
| OMR | optomotor response |
References
- de Coster, S.; van Larebeke, N. Endocrine-Disrupting Chemicals: Associated Disorders and Mechanisms of Action. J. Environ. Public Health 2012, 2012, 1–52. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Mukai, M. Endocrine Disruption in the Context of Life Cycles: Perception and Transduction of Environmental Cues. Gen. Comp. Endocrinol. 2009, 163, 92–96. [Google Scholar] [CrossRef]
- Soffker, M.; Tyler, C. Endocrine Disrupting Chemicals and Sexual Behaviors in Fish–A Critical Review on Effects and Possible Consequences. Crit. Rev. Toxicol. 2012, 42, 653–668. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Health Sciences. Endocrine Disruptors and Your Health; US Department of Health and Human Services: Research Triangle Park, NC, USA, 2020; pp. 1–2.
- Ben-Jonathan, N.; Steinmetz, R. Xenoestrogens: The Emerging Story of Bisphenol A. Trends Endocrinol. Metab. 1998, 9, 124–128. [Google Scholar] [CrossRef]
- Kolpin, D.; Furlong, E.; Meyer, M.; Thurman, M.; Zaugg, S.; Barber, L.; Herbert, B. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.R. Sources of Estrogen and Their Importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Dickerson, S.M.; Cunningham, S.L.; Patisaul, H.B.; Woller, M.J.; Gore, A.C. Endocrine Disruption of Brain Sexual Differentiation by Developmental PCB Exposure. Endocrinology. 2011, 152, 581–594. [Google Scholar] [CrossRef] [Green Version]
- Jašarević, E.; Sieli, P.T.; Twellman, E.E.; Welsh, T.H.; Schachtman, T.R.; Roberts, R.M.; Geary, D.C.; Rosenfeld, C.S. Disruption of Adult Expression of Sexually Selected Traits by Developmental Exposure to Bisphenol A. Proc. Natl. Acad. Sci. USA 2011, 108, 11715–11720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, A.; Kluk, M.; Fox, J.; Park, M.; Turner, J.E. The Effects of Aromatase Inhibitors and Selective Estrogen Receptor Modulators on Eye Development in the Zebrafish (Danio Rerio). Curr. Eye Res. 2007, 32, 819–827. [Google Scholar] [CrossRef]
- Dong, W.; Muramoto, W.; Nagai, Y.; Takehana, K.; Stegeman, J.J.; Teraoka, H.; Hiraga, T. Retinal Neuronal Cell Is a Toxicological Target of Tributyltin in Developing Zebrafish. J. Vet. Med. Sci. 2006, 68, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Huang, B. TBT ( Tributyltin ) Toxicity to the Visual and Olfactory Functions Of. Zool. Stud. 1999, 38, 189–195. [Google Scholar]
- Hano, T.; Oshima, Y.; Kim, S.G.; Satone, H.; Oba, Y.; Kitano, T.; Inoue, S.; Shimasaki, Y.; Honjo, T. Tributyltin Causes Abnormal Development in Embryos of Medaka, Oryzias Latipes. Chemosphere 2007, 69, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Cascio, C.; Deidda, I.; Russo, D.; Guarneri, P. The Estrogenic Retina: The Potential Contribution to Healthy Aging and Age-Related Neurodegenerative Diseases of the Retina. Steroids 2015, 103, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Eisner, A.; Luoh, S.W. Breast Cancer Medications and Vision: Effects of Treatments for Early-Stage Disease. Curr. Eye Res. 2011, 36, 867–885. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Chatziralli, I.P.; Zagouri, F.; Zografos, G.C. Macular Oedema Due to Letrozole: A First Case Report. Clin. Exp. Optom. 2012, 95, 646–650. [Google Scholar] [CrossRef]
- Rinkwitz, S.; Mourrain, P.; Becker, T.S. Zebrafish: An Integrative System for Neurogenomics and Neurosciences. Prog. Neurobiol. 2011, 93, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, J.; Saszik, S.; Givin, C.M.; Hardesty, H.R.; Sutherland, S.E. Effects of Embryonic Exposure to Ethanol on Zebrafish Visual Function. Neurotoxicol. Teratol. 2002, 24, 759–766. [Google Scholar] [CrossRef]
- Richards, F.M.; Alderton, W.K.; Kimber, G.M.; Liu, Z.; Strang, I.; Redfern, W.S.; Valentin, J.P.; Winter, M.J.; Hutchinson, T.H. Validation of the Use of Zebrafish Larvae in Visual Safety Assessment. J. Pharmacol. Toxicol. Methods. 2008, 58, 50–58. [Google Scholar] [CrossRef]
- Canestro, C.; Yokoi, H.; Postlethwait, J.H. Evolutionary Developmental Biology and Genomics. Nat. Rev. Genet. 2007, 8, 932–943. [Google Scholar] [CrossRef]
- Kishida, M.; McLellan, M.; Miranda, J.A.; Callard, G.V. Estrogen and Xenoestrogens Upregulate the Brain Aromatase Isoform (P450aromB) and Perturb Markers of Early Development in Zebrafish (Danio Rerio). Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2001, 129, 261–268. [Google Scholar] [CrossRef]
- Link, B.A.; Collery, R.F. Zebrafish Models of Retinal Disease. Annu. Rev. Vis. Sci. 2015, 1, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.A.; Dowling, J.E. Early-Eye Morphogenesis in the Zebrafish, Brachydanio Rerio. J. Comp. Neurol. 1994, 344, 532–542. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Dowling, J.E. Early Retinal Development in the Zebrafish, Danio Rerio: Light and Electron Microscopic Analyses. J. Comp. Neurol. 1999, 404, 515–536. [Google Scholar] [CrossRef]
- Stuermer, C.A.O. Retinotopic Organization of the Developing Retinotectal Projection in the Zebrafish Embryo. J. Neurosci. 1988, 8, 4513–4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houser, A.; McNair, C.; Piccinini, R.; Luxhoj, A.; Bell, W.E.; Turner, J.E. Effects of Estrogen on the Neuromuscular System in the Embryonic Zebrafish (Danio Rerio). Brain Res. 2011, 1381, 106–116. [Google Scholar] [CrossRef]
- Lassiter, C.S.; Linney, E. Embryonic Expression and Steroid Regulation of Brain Aromatase Cyp19a1b in Zebrafish (Danio Rerio). Zebrafish 2007, 4, 49–57. [Google Scholar] [CrossRef]
- Weber, D.N.; Connaughton, V.P.; Dellinger, J.A.; Klemer, D.; Udvadia, A.; Carvan, M.J. Selenomethionine Reduces Visual Deficits Due to Developmental Methylmercury Exposures. Physiol. Behav. 2008, 93, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Bahadori, R.; Huber, M.; Rinner, O.; Seeliger, M.W.; Geiger-Rudolph, S.; Geisler, R.; Neuhauss, S.C.F. Retinal Function and Morphology in Two Zebrafish Models of Oculo-Renal Syndromes. Eur. J. Neurosci. 2003, 18, 1377–1386. [Google Scholar] [CrossRef]
- Avanesov, A.; Malicki, J. Approaches to Study Neurogenesis in the Zebrafish Retina. Methods Cell Biol. 2004, 2004, 333–384. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to Bisphenol A and 4-Tertiary-Octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.R.; Schoenfelder, G. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakind, J.S.; Naiman, D.Q. Daily Intake of Bisphenol A and Potential Sources of Exposure: 2005–2006 National Health and Nutrition Examination Survey. J. Expo. Sci. Environ. Epidemiol. 2010, 21, 272–279. [Google Scholar] [CrossRef]
- Chapin, R.E.; Adams, J.; Boekelheide, K.; Earl Gray, L., Jr.; Hayward, S.W.; Lees, P.S.J.; McIntyre, B.S.; Portier, K.M.; Schnorr, T.M.; Selevan, S.G.; et al. NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Bisphenol A. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2008, 83, 157–395. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-a and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, O.; Oishi, S. Disposition of Orally Administered 2,2-Bis94-Hydroxyphenyl)Propane (Bisphenol A) in Pregnant Rats and the Placental Transfer to Fetuses. Environ. Health Perspect. 2000, 108, 931–935. [Google Scholar] [CrossRef]
- Schonfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent Bisphenol A Accumulation in the Human Maternal-Fetal-Placental Unit. Environ. Health Perspect. 2002, 110, A703–A707. [Google Scholar] [CrossRef]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental Chemicals in Pregnant Women in the United States: NHANES 2003– 2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [Green Version]
- Farabollini, F.; Porrini, S.; Dessi-Fulgherit, F. Perinatal Exposure to the Estrogenic Pollutant Bisphenol A Affects Behavior in Male and Female Rats. Pharmacol. Biochem. Behav. 1999, 64, 687–694. [Google Scholar] [CrossRef]
- Kim, M.E.; Park, H.R.; Gong, E.J.; Choi, S.Y.; Kim, H.S.; Lee, J. Exposure to Bisphenol A Appears to Impair Hippocampal Neurogenesis and Spatial Learning and Memory. Food Chem. Toxicol. 2011, 49, 3383–3389. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Edwards, M.; Shetty, S.R.J.; Gatewood, J.D.; Taylor, J.A.; Rissman, E.F.; Connelly, J.J. Gestational Exposure to Bisphenol A Produces Transgenerational Changes in Behaviors and Gene Expression. Endocrinology 2012, 153, 3828–3838. [Google Scholar] [CrossRef] [Green Version]
- Hinfray, N.; Palluel, O.; Turies, C.; Cousin, C.; Porcher, J.M.; Brion, F. Brain and Gonadal Aromatase as Potential Targets of Endocrine Disrupting Chemicals in a Model Species, the Zebrafish (Danio Rerio). Environ. Toxicol. 2006, 21, 332–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, A.C.; Ramsdell, J.S. Sexual Dimorphism of Brain Aromatase Activity in Medaka: Induction of a Female Phenotype by Estradiol. Environ. Health Perspect. 2001, 109, 257–264. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Qiu, W.; Zhao, Y.; Yang, M.; Farajzadeh, M.; Pan, C.; Wayne, N.L. Actions of Bisphenol A and Bisphenol S on the Reproductive Neuroendocrine System during Early Development in Zebrafish. Endocrinology 2016, 157, 636–647. [Google Scholar] [CrossRef] [Green Version]
- Saili, K.S.; Corvi, M.M.; Weber, D.N.; Patel, A.U.; Das, S.R.; Przybyla, J.; Anderson, K.A.; Tanguay, R.L. Neurodevelopmental Low-Dose Bisphenol A Exposure Leads to Early Life-Stage Hyperactivity and Learning Deficits in Adult Zebrafish. Toxicology. 2012, 291, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, D.; Hoffmann, R.; Hoke, E.S.; Tanguay, R.L. Bisphenol a Exposure During Early Development Induces Sex-Specific Changes in Adult Zebrafish. J. Toxicol Env. Heal. 2015, 78, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Dong, Q.; Chen, Y.; Jiang, H.; Xiao, Q.; Wang, Y.; Li, W.; Bai, C.; Huang, C.; Yang, D. Bisphenol A Affects Axonal Growth, Musculature and Motor Behavior in Developing Zebrafish. Aquat. Toxicol. 2013, 142–143, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.K.F.; Yeung, B.H.Y.; Wan, H.T.; Wong, C.K.C. Early Embryogenesis in Zebrafish Is Affected by Bisphenol A Exposure. Biol. Open. 2013, 2, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibert, Y.; Sassi-Messai, S.; Fini, J.B.; Bernard, L.; Zalko, D.; Cravedi, J.P.; Balaguer, P.; Andersson-Lendahl, M.; Demeneix, B.; Laudet, V. Bisphenol A Induces Otolith Malformations during Vertebrate Embryogenesis. BMC Dev. Biol. 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Roehlicher, M.; Liedtke, A.; Groh, K.; López-Schier, H.; Neuhauss, S.C.F.; Segner, H.; Eggen, R.I.L. Estrogen Receptor Subtype Β2 Is Involved in Neuromast Development in Zebrafish (Danio Rerio) Larvae. Dev. Biol. 2009, 330, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, L.; Sheth, M.; Young, A.; Kruger, M.; Wayman, G.A.; Coffin, A.B. The Effect of the Aquatic Contaminants Bisphenol-A and PCB-95 on the Zebrafish Lateral Line. Neurotoxicology. 2015, 46, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Lawrence, C.; Lawrence, C. The Husbandry of Zebrafish (Danio Rerio): A Review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Muto, A.; Orger, M.B.; Wehman, A.M.; Smear, M.C.; Kay, J.N.; Page-McCaw, P.S.; Gahtan, E.; Xiao, T.; Nevin, L.M.; Gosse, N.J.; et al. Forward Genetic Analysis of Visual Behavior in Zebrafish. PLoS Genet. 2005, 1, e66. [Google Scholar] [CrossRef] [PubMed]
- Le Page, Y.; Vosges, M.; Servili, A.; Brion, F.; Kah, O. Neuroendocrine Effects of Endocrine Disruptors in Teleost Fish. J. Toxicol. Environ. Health Part B 2011, 14, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Cano-Nicolau, J.; Vaillant, C.; Pellegrini, E.; Charlier, T.D.; Kah, O.; Coumailleau, P. Estrogenic Effects of Several BPA Analogs in the Developing Zebrafish Brain. Front. Neurosci. 2016, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kinch, C.D.; Ibhazehiebo, K.; Jeong, J.; Habibi, H.R.; Kurrasch, D.M. Low-Dose Exposure to Bisphenol A and Replacement Bisphenol S Induces Precocious Hypothalamic Neurogenesis in Embryonic Zebrafish. Proc. Natl. Acad. Sci. USA 2015, 112, 1475–1480. [Google Scholar] [CrossRef] [Green Version]
- Santangeli, S.; Maradonna, F.; Gioacchi, G.; Cobellis, G.; Piccinetti, C.C.; Valle, L.D.; Carnevali, O. BPA-Induced Deregulation Of Epigenetic Patterns: Effects On Female Zebrafish Reproduction. Nat. Publ. Gr. 2016, 1–11. [Google Scholar] [CrossRef]
- Neuhauss, S.C.F. Behavioral Genetic Approaches to Visual System Development and Function in Zebrafish. J. Neurobiol. 2003, 54, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Portugues, R.; Engert, F. The Neural Basis of Visual Behaviors in the Larval Zebrafish. Curr. Opin. Neurobiol. 2009, 19, 644–647. [Google Scholar] [CrossRef] [Green Version]
- Parker, B.; Connaughton, V.P. Effects of Nicotine on Growth and Development in Larval Zebrafish. Zebrafish 2007, 4, 59–68. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Patterson, J.; Kimmel, R.O. The Development and Behavioral Characteristics of the Startle Response in the Zebra Fish. Dev. Psychobiol. 1974, 7, 47–60. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Sensorimotor Gating in Larval Zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef] [Green Version]
- LeFauve, M.K.; Rowe, C.J.; Crowley-Perry, M.; Wiegand, J.L.; Shapiro, A.G.; Connaughton, V.P. Using a Variant of the Optomotor Response as a Visual Defect Detection Assay in Zebrafish. J. Biol. Methods. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- LeFauve, M.K.; Connaughton, V.P. Developmental Exposure to Heavy Metals Alters Visually-Guided Behaviors in Zebrafish. Curr. Zool. 2017, 63, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Q.; Wong, C.K.C.; Bouwman, R.B.; Wahlstrom, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A Review of Sources, Environmental Levels, and Potential Human Health Impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef]
- Lindholst, C.; Wynne, P.M.; Marriott, P.; Pedersen, S.N.; Bjerregaard, P. Metabolism of Bisphenol A in Zebrafish (Danio Rerio) and Rainbow Trout (Oncorhynchus Mykiss) in Relation to Estrogenic Response. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 169–177. [Google Scholar] [CrossRef]
- Sun, S.X.; Zhang, Y.N.; Lu, D.L.; Wang, W.L.; Mchele, L.S.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Concentration-Dependent Effects of 17B Estradiol and Bisphenol A on Lipid Deposition, Inflammation and Antioxidant Response in Male Zebrafish (Danio Rerio). Chemosphere 2019, 237, 124422. [Google Scholar] [CrossRef]
- Schafers, C.; Teigeler, M.; Wenzel, A.; Maack, G.; Fenske, M.; Segner, H. Concentration- and Time-Dependent Effects of the Synthetic Estrogen 17a-Ethinylestradiol, on Reproductive Capabilities of the Zebrafish, Danio Rerio. J. Toxicol. Environ. Health Part A 2007, 70, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.P.; Hill, R.J., Jr.; Janz, D.M. Developmental Estrogenic Exposure in Zebrafish (Danio Rerio) II. Histological Evaluation of Gametogenesis and Organ Toxicity. Aquat. Toxicol. 2003, 63, 431–446. [Google Scholar] [CrossRef]
- Lyssimachou, A.; Jenssen, B.; Arukwe, A. Brain Cytochrome P450 Aromatase Gene Isoforms and Activity Levels in Atlantic Salmon after Waterborne Exposure to Nominal Environmental Concentrations of Pharmaceutical Ethynylestradiol and Antifoulant Tributyltin. Toxicol. Sci. 2006, 91, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, S.J.; Gerstner, K.A.; Callard, G.V. Real-Time PCR Analysis of Cytochrome P450 Aromatase Expression in Zebrafish: Gene Specific Tissue Distribution, Sex Differences, Developmental Programming, and Estrogen Regulation. Gen. Comp. Endocrinol. 2006, 147, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Mouriec, K.; Pellegrini, E.; Menuet, A.; Adrio, F.; Thieulant, M.L.; Pakdel, F.; Kah, O. Synthesis of Estrogens in Progenitor Cells of Adult Fish Brain: Evolutive Novelty or Exaggeration of a More General Mechanism Implicating Estrogens in Neurogenesis? Brain Res. Bull. 2008, 75, 274–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto-Kazeto, R.; Kight, K.E.; Zohar, Y.; Place, A.R.; Trant, J.M. Localization and Expression of Aromatase MRNA in Adult Zebrafish. Gen. Comp. Endocrinol. 2004, 139, 72–84. [Google Scholar] [CrossRef]
- Chapouton, P.; Skupien, P.; Hesl, B.; Coolen, M.; Moore, J.C.; Madelaine, R.; Kremmer, E.; Faus-Kessler, T.; Blader, P.; Lawson, N.D.; et al. Notch Activity Levels Control the Balance between Quiescence and Recruitment of Adult Neural Stem Cells. J. Neurosci. 2010, 30, 7961–7974. [Google Scholar] [CrossRef] [Green Version]
- Lassiter, C.S.; Kelley, B.; Linney, E. Genomic Structure and Embryonic Expression of Estrogen Receptor Beta a (ERBa) in Zebrafish (Danio Rerio). Gene 2002, 299, 141–151. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Andre, M.; Forgue, J.; Barthe, C.; Babin, P.J. Expression Patterns of Three Estrogen Receptor Genes during Zebrafish (Danio Rerio) Development: Evidence for High Expression in Neuromasts. Gene Expr. Patterns. 2004, 4, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Bardet, P.L.; Horad, B.; Robinson-Rechavi, M.; Laudet, V.; Vanacker, J.M. Characterization of Oestrogen Receptors in Zebrafish (Danio Rerio). J. Mol. Endocrinol. 2002, 28, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Mouriec, K.; Lareyre, J.J.; Tong, S.; Le Page, Y.; Vaillant, C.; Pellegrini, E.; Pakdel, F.; Chung, B.C.; Kah, O.; Anglade, I. Early Regulation of Brain Aromatase (Cyp19a1b) by Estrogen Receptors during Zebrafish Development. Dev. Dyn. 2009, 238, 2641–2651. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Zhu, P.; Li, J.; Sham, K.; Cheng, S.; Li, S.; Zhang, Y.; Cheng, C.; Lin, H. G-Protein-Coupled Estrogen Receptor1 Is Involved in Brain Development during Zebrafish (Danio Rerio) Embryogenesis. Biochem. Biophys. Res. Commun. 2013, 435, 21–27. [Google Scholar] [CrossRef]
- Steinmetz, R.; Brown, N.G.; Allen, D.L.; Bigsby, R.M.; Ben-Jonathan, N. The Environmental Estrogen Bisphenol A Stimulates Prolactin Release in Vitro and in Vivo. Endocrinology 1997, 138, 1780–1786. [Google Scholar] [CrossRef]
- Thomas, P.; Dong, J. Binding and Activation of the Seven-Transmembrane Estrogen Receptor GPR30 by Environmental Estrogens: A Potential Novel Mechanism of Endocrine Disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.A.; Taylor, J.A.; Ruhlen, R.L.; Welshons, W.V.; vom Saal, F.S. Estradiol and Bisphenol A Stimulate Androgen Receptor and Estrogen Receptor Gene Expression in Fetal Mouse Prostate Mesenchyme Cells. Environ. Health Perspect. 2007, 115, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Genco, M.C.; Megrelis, L.; Ruderman, J.V. Effects of Bisphenol A and Triclocarban on Brain-Specific Expression of Aromatase in Early Zebrafish Embryos. Proc. Natl. Acad. Sci. USA 2011, 108, 17732–17737. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.; Niu, L.; Liu, W.; Xu, C. Embryonic Exposure to Butachlor in Zebrafish (Danio Rerio): Endocrine Disruption, Developmental Toxicity and Immunotoxicity. Ecotoxicol. Environ. Saf. 2013, 89, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.J.; Wiegand, J.L.; Connaughton, V.P. Acute Developmental Exposure to 4-Hydroxyandrostenedione Has a Long- Term e Ff Ect on Visually-Guided Behaviors. Neurotoxicol. Teratol. 2017, 64, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascenzi, P.; Bocedi, A.; Marino, M. Structure-Function Relationship of Estrogen Receptor α and β: Impact on Human Health. Mol. Aspects Med. 2006, 27, 299–402. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Marino, M. The Effects of 17β-Estradiol in Cancer Are Mediated by Estrogen Receptor Signaling at the Plasma Membrane. Front. Physiol. 2011, JUN, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Menuet, A.; Pellegrini, E.; Anglade, I.; Blaise, O.; Laudet, V.; Kah, O.; Pakdel, F. Molecular Characterization of Three Estrogen Receptor Forms in Zebrafish: Binding Characteristics, Transactivation Properties, and Tissue Distributions. Biol. Reprod. 2002, 66, 1881–1892. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in Vivo? Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Okada, H.; Tokunaga, T.; Liu, X.; Takayanagi, S.; Matsushima, A.; Shimohigashi, Y. Direct Evidence Revealing Structural Elements Essential for the High Binding Ability of Bisphenol A to Human Estrogen-Related Receptor-γ. Environ. Health Perspect. 2008, 116, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Pellegrini, M.; La Rosa, P.; Acconicia, F. Susceptibility of Estrogen Receptor Rapid Responses to Xenoestrogens: Physiological Outcomes. Steroids 2012, 77, 910–917. [Google Scholar] [CrossRef]
- Sohoni, P.; Sumpter, J.P. Several Environmental Oestrogens Are Also Anti-Androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonefeld-Jorgensen, E.C.; Long, M.; Hofmeister, M.V.; Vinggaard, A.M. Endocrine-Disrupting Potential of Bisphenol A, Bisphenol A Dimethacrylate, 4-n-Nonylphenol, and 4-n-Octylphenol in Vitro: New Data and a Brief Review. Environ. Health Perspect. 2007, 115, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuyu, H.; Nakao, K. Thyroid Hormone Action Is Disrupted by Bisphenol A as an Antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Bansal, R.; Parris, C. Bisphenol-A, an Environmental Contaminant That Acts as a Thyroid Hormone Receptor Antagonist in Vitro, Increases Serum Thyroxine, and Alters RC3/Neurogranin Expression in the Developing Rat Brain. Endocrinology 2005, 146, 607–612. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowley-Perry, M.; Barberio, A.J.; Zeino, J.; Winston, E.R.; Connaughton, V.P. Zebrafish Optomotor Response and Morphology Are Altered by Transient, Developmental Exposure to Bisphenol-A. J. Dev. Biol. 2021, 9, 14. https://0-doi-org.brum.beds.ac.uk/10.3390/jdb9020014
Crowley-Perry M, Barberio AJ, Zeino J, Winston ER, Connaughton VP. Zebrafish Optomotor Response and Morphology Are Altered by Transient, Developmental Exposure to Bisphenol-A. Journal of Developmental Biology. 2021; 9(2):14. https://0-doi-org.brum.beds.ac.uk/10.3390/jdb9020014
Chicago/Turabian StyleCrowley-Perry, Mikayla, Angelo J. Barberio, Jude Zeino, Erica R. Winston, and Victoria P. Connaughton. 2021. "Zebrafish Optomotor Response and Morphology Are Altered by Transient, Developmental Exposure to Bisphenol-A" Journal of Developmental Biology 9, no. 2: 14. https://0-doi-org.brum.beds.ac.uk/10.3390/jdb9020014