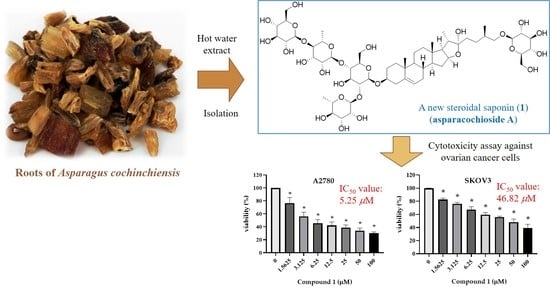
A Novel Cytotoxic Steroidal Saponin from the Roots of Asparagus cochinchinensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Compound 1
2.2. The Cytotoxicity of the Compounds Isolated from A. cochinchinensis against Human Ovarian Cancer Cells
2.3. Induction of Apoptotic Cell Death by Asparacochioside A (1) in Human Ovarian Cancer Cells
3. Materials and Methods
3.1. Plant Material
3.2. General Experimental Procedures
3.3. Isolation of Compounds 1−7
Asparacochioside A (1)
3.4. Acidic Hydrolysis of Compound 1
3.5. Absolute Configurations of the β-Glucose and α-Rhamnose in Compound 1
3.6. Cell Culture
3.7. MTT Assay
3.8. Annexin V−FITC Staining Assay for Apoptosis Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Negi, J.S.; Singh, P.; Joshi, G.P.; Rawat, M.S.; Bisht, V.K. Chemical constituents of Asparagus. Phamacogn. Rev. 2010, 4, 215–220. [Google Scholar]
- Xiong, D.; Yu, L.X.; Yan, X.; Guo, C.; Xiong, Y. Effects of root and stem extracts of Asparagus cochinchinensis on biochemical indicators related to aging in the brain and liver of mice. Am. J. Chin. Med. 2011, 39, 719–726. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, C.L.; Xuan, W.D.; Li, H.L.; Liu, R.H.; Xu, X.K.; Chen, H.S. A new furostanol saponin from Asparagus cochinchinensis. Arch. Pharm. Res. 2011, 34, 1587–1591. [Google Scholar] [CrossRef]
- Guo-Lei, Z.H.U.; Qian, H.A.O.; Rong-Tao, L.I.; Hai-Zhou, L.I. Steroidal saponins from the roots of Asparagus cochinchinensis. Chin. J. Nat. Med. 2014, 12, 213–217. [Google Scholar]
- Le Son, H.; Anh, N.P. Phytochemical composition, in vitro antioxidant and anticancer activities of quercetin from methanol extract of Asparagus cochinchinensis (Lour.) Merr. tuber. J. Med. Plant. Res. 2013, 7, 3360–3366. [Google Scholar]
- Samad, N.B.; Debnath, T.; Abul Hasnat, M.; Pervin, M.; Kim, D.H.; Jo, J.E.; Park, S.R.; Lim, B.O. Phenolic Contents, Antioxidant and Anti-inflammatory Activities of Asparagus cochinchinensis (Loureiro) Merrill. J. Food Biochem. 2014, 38, 83–91. [Google Scholar] [CrossRef]
- Park, M.; Cheon, M.S.; Kim, S.H.; Chun, J.M.; Lee, A.Y.; Moon, B.C.; Yoon, T.; Choo, B.K.; Kim, H.K. Anticancer activity of Asparagus cochinchinensis extract and fractions in HepG2 cells. J. Korean Soc. Appl. Bio Chem. 2011, 54, 188–193. [Google Scholar] [CrossRef]
- Zhou, M.; Huang, L.; Li, L.; Wei, Y.; Shu, J.; Liu, X.; Huang, H. New furostanol saponins with anti-inflammatory and cytotoxic activities from the rhizomes of Smilax davidiana. Steroids 2017, 127, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yu, Y.M.; Qi, Q.L.; Wu, X.D.; Wang, J.; Tang, S.A. Steroidal saponins from the rhizome of Polygonatum sibiricum. J. Asian. Nat. Prod. Res. 2019, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Dong, A.; Yao, X.; Kobayashi, H.; Iwasaki, S. Antineoplastic agents II: Four furostanol glycosides from rhizomes of Dioscorea collettii var. hypoglauca. Planta Med. 1997, 63, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.L.; Hao, Q.; Xing, L.; Yang, X.Q.; Xie, S.D.; Zhao, P.; Li, H.Z. C21, C22 pregnane glycosides and cytotoxic C27 spriostanol steroids from Asparagus cochinchinesis. Steroids 2021, 172, 108874. [Google Scholar] [CrossRef]
- Singh, S.B.; Thakur, R.S. Costusoside-I and costusoside-J, two new furostanol saponins from the seeds of Costus speciosus. Phytochemistry 1982, 21, 911–915. [Google Scholar] [CrossRef]
- Agrawal, P.K. Dependence of 1H NMR chemical shifts of geminal protons of glycosyloxy methylene (H2-26) on the orientation of the 27-methyl group of furostane-type steroidal saponins. Magn. Reson. Chem. 2004, 42, 990–993. [Google Scholar] [CrossRef]
- Challinor, V.L.; Piacente, S.; De Voss, J.J. NMR assignment of the absolute configuration of C-25 in furostanol steroidal saponins. Steroids 2012, 77, 602–608. [Google Scholar] [CrossRef]
- Ju, Y.; Jia, Z.J. Steroidal saponins from the rhizomes of Smilax menispermoidea. Phytochemistry 1992, 31, 1349–1351. [Google Scholar] [CrossRef]
- Shi, J.G.; Li, G.Q.; Huang, S.Y.; Mo, S.Y.; Wang, Y.; Yang, Y.C.; Hu, W.Y. Furostanol oligoglycosides from Asparagus cochinchinensis. J. Asian Nat. Prod. Res. 2004, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Liu, R.H.; Shao, F. Structural determination of two new steroidal saponins from Smilax china. Magn. Reson. Chem. 2009, 47, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, A.; Kintya, P.; Wyrzykiewicz, B.; Gorincioi, E. Steroidal glycosides from Veronica chamaedrys L. Part I. The structures of chamaedrosides C, C1, C2, E, E1 and E2. Nat. Prod. Commun. 2012, 7, 565–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sautour, M.; Mitaine-Offer, A.C.; Lacaille-Dubois, M.A. The Dioscorea genus: A review of bioactive steroid saponins. J. Nat. Med. 2007, 61, 91–101. [Google Scholar] [CrossRef]
- Tanaka, T.; Tatsuya, N.; Toshihisa, U.; Kenji, T.; Isao, K. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]

 ) and HMBC (
) and HMBC ( ) correlations of compound 1 (A). Key 1H−1H NOESY (
) correlations of compound 1 (A). Key 1H−1H NOESY ( : blue dashed arrows) correlations of compound 1 (B).
: blue dashed arrows) correlations of compound 1 (B).
 ) and HMBC (
) and HMBC ( ) correlations of compound 1 (A). Key 1H−1H NOESY (
) correlations of compound 1 (A). Key 1H−1H NOESY ( : blue dashed arrows) correlations of compound 1 (B).
: blue dashed arrows) correlations of compound 1 (B).


| Position | 1 | Protodioscin (2) a | ||
|---|---|---|---|---|
| δH Multi (J in Hz) | δC | δH Multi (J in Hz) | δC | |
| 1 | 1.85, 1H, m/2.07, 1H, m | 30.1 | 1.86, 1H, m/2.08, 1H, m | 29.8 |
| 2 | 1.44, 2H, m | 21.0 | 1.54, 2H, m | 20.9 |
| 3 | 3.88, 1H, d (11.0, 6.5) | 78.0 | 3.81, 1H b | 78.4 |
| 4 | 2.73, 1H, d (11.0)/2.79, 1H, d (10.5) | 38.9 | 2.74, 1H, d (11.5)/2.79, 1H, d (10.5) | 38.8 |
| 5 | - | 140.7 | - | 140.6 |
| 6 | 5.30, 1H, s | 121.8 | 5.34, 1H, s | 121.8 |
| 7 | 1.88, 2H, m | 32.2 | 1.86, 2H, m | 32.2 |
| 8 | 1.57, 1H, m | 31.6 | 1.56, 1H, m | 31.5 |
| 9 | 0.90, 1H b | 50.3 | 0.90, 1H b | 50.1 |
| 10 | - | 37.1 | - | 37.1 |
| 11 | 0.98, 1H b/1.32, 1H b | 37.4 | 0.97, 1H b/1.30, 1H b | 37.8 |
| 12 | 1.12, 1H, m/1.74, 1H, m | 39.8 | 1.12, 1H, m/1.75, 1H, m | 39.7 |
| 13 | - | 40.7 | - | 40.6 |
| 14 | 1.07, 1H b | 56.6 | 1.07, 1H b | 56.4 |
| 15 | 1.48, 1H b / 2.02, 1H b | 32.4 | 1.48, 1H, m/2.03, 1H, m | 32.3 |
| 16 | 4.96, 1H, t (4.5) | 81.0 | 4.46, 1H, m | 81.1 |
| 17 | 1.93, 1H b | 63.7 | 1.94, 1H b | 63.7 |
| 18 | 1.08. 3H, s | 19.3 | 1.05, 3H, s | 19.2 |
| 19 | 0.89, 3H, s | 16.4 | 0.89, 3H, s | 16.3 |
| 20 | 2.25, 1H, q (2.0) | 40.6 | 2.24, 1H, m | 40.5 |
| 21 | 1.34, 3H, d (7.0) | 16.4 | 1.34, 3H, d (7.0) | 16.3 |
| 22 | - | 110.6 | - | 111.0 |
| 23 | 2.07, 2H b | 37.1 | 1.57, 2H, dd (10.5, 5.0) | 30.0 |
| 24 | 1.68, 1H, d (4.0)/2.05, 1H, m | 28.3 | 1.68, 1H, d (4.0)/2.05, 1H, m | 28.2 |
| 25 | 1.94, 1H b | 34.2 | 1.96, 1H b | 34.1 |
| 26 | 3.63, 1H, dd (9.5, 6.0)/3.95, 1H, dd (9.0, 6.5) | 78.5 | 3.63, 1H, d (4.0)/3.99, 1H, d (6.5) | 78.4 |
| 27 | 0.99, 3H, d (6.5) | 17.4 | 0.99, 3H, d (6.0) | 17.3 |
| Position | 1 | Protodioscin (2) a | ||
|---|---|---|---|---|
| δH Multi (J in Hz) | δC | δH Multi (J in Hz) | δC | |
| 3-O-Glc-1’ | 4.95, 1H, d (7.5) | 100.3 | 4.96, 1H, d (7.0) | 100.1 |
| 2’ | 3.88, 1H b | 78.0 | 3.78, 1H b | 77.9 |
| 3’ | 3.63, 1H b | 76.9 | 3.61, 1H b | 76.8 |
| 4’ | 3.95, 1H b | 78.4 | 3.92, 1H b | 78.3 |
| 5’ | 4.43, 1H b | 77.3 | 4.40, 1H b | 77.5 |
| 6’ | 4.06, 1H, t (8.0)/4.20, 1H b | 61.1 | 4.04, 1H, t (8.0)/4.23, 1H b | 61.1 |
| 2’-O-Rha-1” | 6.42, 1H, s | 101.9 | 6.41, 1H, s | 101.9 |
| 2” | 4.67, 1H b | 71.8 | 4.61, 1H b | 72.4 |
| 3” | 4.87, 1H, s | 72.5 | 4.75, 1H, s | 72.6 |
| 4” | 4.37, 1H b | 74.00 | 4.34, 1H b | 73.8 |
| 5” | 5.04, 1H b | 69.5 | 4.95, 1H b | 69.4 |
| 6” | 1.77, 3H, d (6.0) | 18.3 | 1.76, 3H, d (6.0) | 18.4 |
| 4’-O-Rha-1’” | 5.87, 1H, s | 102.0 | 5.87, 1H, s | 102.7 |
| 2’” | 4.64, 1H, dd (9.0, 3.0) | 72.4 | 4.61, 1H, dd (9.0, 3.0) | 72.4 |
| 3’” | 4.65, 1H, d (4.5) | 72.8 | 4.61, 1H, m | 72.7 |
| 4’” | 4.21, 1H b | 74.01 | 4.61, 1H b | 73.3 |
| 5’” | 4.96, 1H b | 71.2 | 4.92, 1H b | 70.2 |
| 6’” | 1.67, 3H, d (6.0) | 18.6 | 1.64, 3H, d (6.0) | 18.5 |
| 26-O-Glc-1”“ | 4.81, 1H, d (7.5) | 104.8 | 4.87, 1H, d (7.5) | 104.8 |
| 2”“ | 4.14, 1H, t (8.5) | 75.1 | 4.14, 1H, t (8.5) | 75.0 |
| 3”“ | 3.84, 1H b | 77.9 | 3.85, 1H, m | 77.8 |
| 4”“ | 4.24, 1H b | 71.6 | 4.23, 1H b | 71.5 |
| 5”“ | 4.29, 1H, t (9.5) | 78.5 | 4.30, 1H, t (9.0) | 78.4 |
| 6”“ | 4.36, 1H b/4.40, 1H b | 62.7 | 4.36, 1H b/4.41, 1H b | 62.6 |
| 4’”-O-Glc-1”“‘ | 5.24, 1H, d (7.5) | 106.7 | ||
| 2”“‘ | 4.03, 1H, m | 76.5 | ||
| 3”“‘ | 3.63, 1H, m/3.95, 1H, m | 75.3 | ||
| 4”“‘ | 4.23, 1H b | 78.5 | ||
| 5”“‘ | 4.30, 1H, t (9.0) | 71.2 | ||
| 6”“‘ | 4.37, 1H b 4.51/1H, d (11.5) | 62.3 | ||
| Compound | IC50 (μM) a | |
|---|---|---|
| A2780 | SKOV3 | |
| 1 | 5.25 ± 2.2d,e,f | 46.82 ± 9.43 f |
| 2 | 10.14 ± 0.12c, e | >100 |
| 3 | 21.78 ± 8.14 c,d,e | >100 |
| Cisplatin b | 10.82 ± 0.43 c,e | 17.55 ± 4.46c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Choi, H.Y.; Kim, H.M.; Choi, J.-H.; Jang, D.S. A Novel Cytotoxic Steroidal Saponin from the Roots of Asparagus cochinchinensis. Plants 2021, 10, 2067. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10102067
Kim J-Y, Choi HY, Kim HM, Choi J-H, Jang DS. A Novel Cytotoxic Steroidal Saponin from the Roots of Asparagus cochinchinensis. Plants. 2021; 10(10):2067. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10102067
Chicago/Turabian StyleKim, Ji-Young, He Yun Choi, Hye Mi Kim, Jung-Hye Choi, and Dae Sik Jang. 2021. "A Novel Cytotoxic Steroidal Saponin from the Roots of Asparagus cochinchinensis" Plants 10, no. 10: 2067. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10102067