Influence of Light Spectra from LEDs and Scion × Rootstock Genotype Combinations on the Quality of Grafted Watermelon Seedlings
Abstract
:1. Introduction
2. Materials and Methods
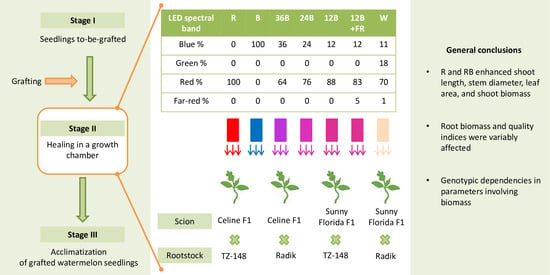
2.1. Plant Material and Growth of Seedlings to Be Grafted
2.2. Grafting, Healing, and Light Conditions in the Healing Chamber
2.3. Sampling and Measurements
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Worldstopexports.com. Available online: http://www.worldstopexports.com/watermelon-exports-by-country/ (accessed on 10 November 2020).
- Lee, S.G. Production of high quality vegetable seedling grafts. Acta Hortic 2007, 759, 169–174. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Odag, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Savvas, D.; Colla, G.; Rouphael, Y.; Schwarz, D. Amelioration of heavy metal and nutrient stress in fruit vegetables by grafting. Sci. Hortic. 2010, 127, 156–161. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Hassell, R.; Levi, A.; King, S.R.; Zhang, X. Grafting effects on vegetable quality. HortScience 2008, 43, 1670–1672. [Google Scholar] [CrossRef] [Green Version]
- Whitelam, G.; Halliday, K. Light and Plant Development; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef] [Green Version]
- Li, X.E.; Liu, F.Y.; Huang, Y.; Kong, Q.S.; Wan, Z.J.; Li, X.; Bie, Z.L. Growth and physiology of grafted watermelon seedlings as affected by different light sources. Acta Hortic 2015, 1086, 59–64. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.S.; Fotelli, M.N.; Kintzonidis, D. Bichromatic red and blue LEDs during healing enhance the vegetative growth and quality of grafted watermelon seedlings. Sci. Hortic. 2020, 109000. [Google Scholar] [CrossRef]
- Lee, J.M.; Oda, M. Grafting of herbaceous vegetable and ornamental crops. In Horticultural Review; Janick, J., Ed.; John Wiley & Sons: New York, NY, USA, 2003; pp. 61–124. [Google Scholar]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic Efficiency and Phytochrome Photoequilibria Determination Using Spectral Data. Trans ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.; Menexes, G.; Dangitsis, C.; Kintzonidis, D. Assessing quantitative criteria for characterization of quality categories for grafted watermelon seedlings. Horticulturae 2019, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Mohr, H.; Kendrick, R.E.; Kronenberg, G.H.M. (Eds.) Photomorphogenesis in Plants, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 353–373. [Google Scholar]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Smith, H.L.; Mcausland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Craver, J.K.; Lopez, R.G. Control of Morphology by Manipulating Light Quality and Daily Light Integral Using LEDs. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016; pp. 203–217. [Google Scholar]
- Madeira, A.C.; Ferreira, A.; De Varennes, A.; Vieira, M.I. SPAD meter versus tristimulus colorimeter to estimate chlorophyll content and leaf color in sweet pepper. Commun. Soil Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Abidi, F.; Girault, T.; Douillet, O.; Guillemain, G.; Sintes, G.; Laffaire, M.; Ahmed, H.B.; Smiti, S.; Huché-Thélier, L.; Leduc, N. Blue light effects on rose photosynthesis and photomorphogenesis. Plant Biol. 2013, 15, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bartucca, M.L.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue:Red LED light proportion affects vegetative parameters, pigment content, and oxidative status of einkorn (Triticum monococcum L. ssp. monococcum) wheatgrass. J. Agric. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef]
- Kasahara, M.; Swartz, T.E.; Olney, M.A.; Onodera, A.; Mochizuki, N.; Fukuzawa, H.; Asamizu, E.; Tabata, S.; Kanegae, H.; Takano, M.; et al. Photochemical properties of the flavin mononucleotidebinding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii. Plant Physiol. 2002, 129, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose−responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, J.; Emami, S. Response of tomato and pepper transplants to light spectra provided by light emitting diodes. Int. J. Veg. Sci. 2013, 19, 138–149. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Cope, K.R.; Bugbee, B. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. Hortscience 2013, 48, 504–509. [Google Scholar] [CrossRef]
- Folta, K.M.; Lieg, E.J.; Durham, T.; Spalding, E.P. Primary inhibition of hypocotyl growth and phototropism depend differently on phototropin-mediated increases in cytoplasmic calcium induced by blue light. Plant Physiol. 2003, 133, 1464–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougher, T.A.; Bugbee, B. Differences in the response of wheat, soybean and lettuce to reduced blue radiation. Photochem. Photobiol. 2001, 73, 199–207. [Google Scholar] [CrossRef]
- Tsukaya, H. Genetic evidence for polarities that regulate leaf morphogenesis. J. Plant Res. 1998, 111, 113–119. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenbaek, M.; Tybirk, E.; Kristensen, H.L. Glucosinolate and carotenoid content of white- and yellow-flowering rapeseed grown for human consumption as sprouts and seedlings under light emitting diodes. Eur. Food Res. Technol. 2018, 244, 1121–1131. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. Improvement in drought tolerance of lemon balm, Melissa officinalis L. under the pre-treatment of LED lighting. Plant Physiol. Biochem. 2019, 139, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.J.; Norris, J.R., Jr. Chlorophyll and light energy transduction in photosynthesis. Curr. Top Bioenerg. 1973, 41–75. [Google Scholar]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red- and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult. 2008, 92, 147–153. [Google Scholar] [CrossRef]
- Ouzounis, T.; Heuvelink, E.; Ji, H.; Schouten, H.J.; Visser, R.G.F.; Marcelis, L.F.M. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. Acta Hortic. 2016, 1134, 251–258. [Google Scholar] [CrossRef]
- Rabara, R.C.; Behrman, G.; Timbol, T.; Rushton, P.J. Effect of spectral quality of monochromatic LED lights on the growth of artichoke seedlings. Front. Plant Sci. 2017, 8, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Waveband | Light Treatment | ||||||
|---|---|---|---|---|---|---|---|
| R | B | 36B | 24B | 12B | 12B+FR | W | |
| UV %; 380–399 nm | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blue %; 400–499 nm | 0 | 100 | 36 | 24 | 12 | 12 | 11 |
| Green %; 500–599 nm | 0 | 0 | 0 | 0 | 0 | 0 | 18 |
| Red %; 600–699 nm | 100 | 0 | 64 | 76 | 88 | 83 | 70 |
| Far-red %; 700–780 nm | 0 | 0 | 0 | 0 | 0 | 5 | 1 |
| YPF (μmol m−2 s−1) | 79.1 | 63.8 | 73.5 | 75.4 | 77.2 | 73.9 | 75.1 |
| PPS | 0.89 | 0.51 | 0.88 | 0.89 | 0.89 | 0.88 | 0.89 |
| Light Treatment | Shoot Length (mm) | R/S Ratio | Chl Content | DW/L × 1000 | DQI × 1000 |
|---|---|---|---|---|---|
| Celine F1 × TZ-148 | |||||
| R | 31.23 ± 1.68 b | 0.20 ± 0.02 a | 27.48 ± 1.58 a | 4.93 ± 0.32 a | 14.29 ± 1.09 abc |
| B | 36.31 ± 1.40 ab | 0.18 ± 0.02 a | 28.55 ± 1.79 a | 3.91 ± 0.12 cd | 11.15 ± 0.73 c |
| 36B | 39.75 ± 1.27 a | 0.18 ± 0.01 a | 30.73 ± 2.93 a | 3.73 ± 0.21 d | 11.98 ± 1.16 bc |
| 24B | 36.41 ± 0.78 ab | 0.17 ± 0.01 a | 29.18 ± 1.26 a | 4.06 ± 0.26 cd | 11.16 ± 1.38 c |
| 12B | 33.78 ± 0.99 b | 0.19 ± 0.01 a | 32.39 ± 1.99 a | 4.82 ± 0.24 ab | 15.78 ± 1.21 a |
| 12B+FR | 33.96 ± 1.03 b | 0.23 ± 0.02 a | 28.08 ± 2.41 a | 4.58 ± 0.29 abc | 15.25 ± 0.60 ab |
| W | 33.86 ± 1.75 b | 0.21 ± 0.03 a | 28.08 ± 1.66 a | 4.58 ± 0.21 abc | 14.15 ± 1.56 abc |
| Sunny Florida F1 × TZ-148 | |||||
| R | 31.55 ± 2.24 b | 0.26 ± 0.01 a | 28.95 ± 1.66 a | 5.07 ± 0.42 a | 18.28 ± 1.50 a |
| B | 33.47 ± 1.15 b | 0.21 ± 0.02 ab | 23.84 ± 2.08 a | 4.27 ± 0.20 bc | 13.44 ± 0.91 b |
| 36B | 35.49 ± 1.16 ab | 0.19 ± 0.02 ab | 29.54 ± 1.68 a | 4.40 ± 0.28 abc | 13.28 ± 1.81 b |
| 24B | 41.46 ± 1.57 a | 0.19 ± 0.02 ab | 30.43 ± 1.74 a | 3.97 ± 0.20 c | 12.05 ± 0.52 b |
| 12B | 31.44 ± 1.01 b | 0.17 ± 0.01 b | 28.71 ± 1.82 a | 4.21 ± 0.25 bc | 10.48 ± 0.56 b |
| 12B+FR | 33.01 ± 1.41 b | 0.18 ± 0.01 b | 26.78 ± 1.48 a | 4.76 ± 0.21 ab | 12.68 ± 0.68 b |
| W | 33.37 ± 0.86 b | 0.18 ± 0.02 b | 26.23 ± 0.94 a | 4.26 ± 0.12 bc | 11.78 ± 0.95 b |
| Celine F1 × Radik | |||||
| R | 32.05 ± 1.35 b | 0.09 ± 0.03 a | 26.11 ± 1.35 bc | 4.18 ± 0.14 b | 10.93 ± 0.39 bc |
| B | 36.58 ± 1.98 ab | 0.11 ± 0.03 a | 20.79 ± 1.58 c | 3.78 ± 0.25 bc | 11.99 ± 0.66 abc |
| 36B | 33.67 ± 1.04 ab | 0.13 ± 0.04 a | 25.48 ± 1.67 bc | 3.93 ± 0.13 bc | 11.82 ± 0.48 abc |
| 24B | 31.08 ± 1.20 b | 0.11 ± 0.03 a | 36.10 ± 2.87 a | 4.92 ± 0.29 a | 13.43 ± 0.72 a |
| 12B | 34.65 ± 1.19 ab | 0.11 ± 0.03 a | 29.29 ± 1.35 ab | 4.25 ± 0.13 b | 11.49 ± 0.84 bc |
| 12B+FR | 39.26 ± 1.46 a | 0.12 ± 0.04 a | 28.30 ± 1.77 bc | 3.47 ± 0.19 c | 10.52 ± 0.75 c |
| W | 31.91 ± 1.47 b | 0.12 ± 0.04 a | 26.69 ± 1.27 bc | 4.31 ± 0.25 b | 12.72 ± 0.59 ab |
| Sunny Florida F1 × Radik | |||||
| R | 31.79 ± 1.59 a | 0.20 ± 0.01 a | 28.68 ± 1.97 a | 4.59 ± 0.25 a | 15.08 ± 1.12 a |
| B | 35.02 ± 1.05 a | 0.19 ± 0.02 a | 23.61 ± 1.06 a | 3.99 ± 0.24 b | 13.19 ± 0.93 ab |
| 36B | 34.57 ± 0.91 a | 0.19 ± 0.02 a | 28.45 ± 3.41 a | 4.15 ± 0.07 ab | 12.88 ± 1.00 ab |
| 24B | 36.26 ± 1.24 a | 0.18 ± 0.01 a | 24.50 ± 1.56 a | 4.36 ± 0.18 ab | 13.22 ± 0.54 ab |
| 12B | 33.04 ± 0.66 a | 0.17 ± 0.01 a | 25.95 ± 1.33 a | 4.23 ± 0.12 ab | 11.39 ± 0.70 b |
| 12B+FR | 36.12 ± 0.84 a | 0.18 ± 0.01 a | 30.35 ± 1.65 a | 4.03 ± 0.15 b | 12.06 ± 0.98 b |
| W | 32.62 ± 0.82 a | 0.18 ± 0.01 a | 30.00 ± 1.24 a | 4.27 ± 0.18 ab | 12.80 ± 0.88 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bantis, F.; Dangitsis, C.; Koukounaras, A. Influence of Light Spectra from LEDs and Scion × Rootstock Genotype Combinations on the Quality of Grafted Watermelon Seedlings. Plants 2021, 10, 353. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10020353
Bantis F, Dangitsis C, Koukounaras A. Influence of Light Spectra from LEDs and Scion × Rootstock Genotype Combinations on the Quality of Grafted Watermelon Seedlings. Plants. 2021; 10(2):353. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10020353
Chicago/Turabian StyleBantis, Filippos, Christodoulos Dangitsis, and Athanasios Koukounaras. 2021. "Influence of Light Spectra from LEDs and Scion × Rootstock Genotype Combinations on the Quality of Grafted Watermelon Seedlings" Plants 10, no. 2: 353. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10020353