Response of Tomato Genotypes under Different High Temperatures in Field and Greenhouse Conditions
Abstract
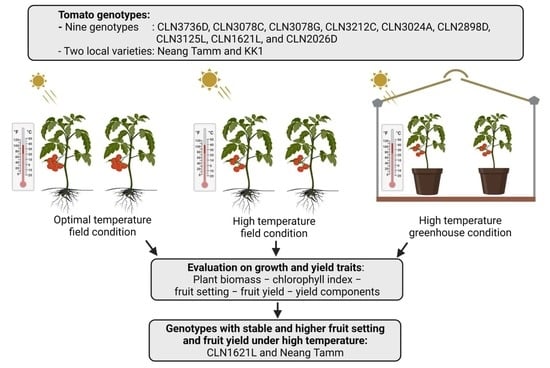
:1. Introduction
2. Results
2.1. Fruit Size Traits
2.2. Fruit Yield Traits
2.3. Chlorophyll Index
2.4. Yield Deviation from OTFC
3. Discussion
4. Materials and Methods
4.1. Experimental Site
4.2. Temperature
4.3. Plant Material
4.4. Experimental Layout and Management
4.4.1. Seedling Preparation
4.4.2. Environment 1: Optimal Temperature Field Condition (OTFC)
4.4.3. Environment 2: High Temperature Field Condition (HTFC)
4.4.4. Environment 3: High Temperature Greenhouse Condition (HTGC)
4.5. Pest Control
4.6. Irrigation
4.7. Chlorophyll Index Reading
4.8. Fruit Harvesting and Data Collection
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OTFC | Optimal Temperature Field Condition |
| HTFC | High Temperature Field Condition |
| HTGC | High Temperature Greenhouse Condition |
| WAP | Weeks After Planting |
| ANOVA | Analysis of Variance |
| G | Genotype |
| C | Condition |
| SD | Standard Deviation |
| HSD | Honest Significant Difference |
| p-value | Probability |
| SFW | Single Fruit Weight |
References
- Wu, F.; Tanksley, S.D. Chromosomal evolution in the plant family Solanaceae. BMC Genom. 2010, 11, 182. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Islam, N.; Sarkar, M.D.; Ali, M.A. Growth and yield of summer tomato as influenced by plant growth regulators. Int. J. Sustain. Agric. 2013, 5, 25–28. [Google Scholar]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 7 November 2019).
- Chhean, S.; Diep, K.; Moustier, P. Vegetable Market Flows and Chains in Phnom Penh. 2004. Available online: https://agritrop.cirad.fr/544875/1/document_544875.pdf (accessed on 7 November 2020).
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Keatinge, J.D.H.; Lin, L.-J.; Ebert, A.W.; Chen, W.Y.; Hughes, J.d.A.; Luther, G.C.; Wang, J.-F.; Ravishankar, M. Overcoming biotic and abiotic stresses in the Solanaceae through grafting: Current status and future perspectives. Biol. Agric. Hortic. 2014, 30, 272–287. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Thoeun, H.C. Observed and projected changes in temperature and rainfall in Cambodia. Weather Clim. Extrem. 2015, 7, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, M.R.; Ayyub, C.M.; Amjad, M.; Waraich, E.A. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions. J. Sci. Food Agric. 2016, 96, 2698–2704. [Google Scholar] [CrossRef]
- Sherzod, R.; Yang, E.Y.; Cho, M.C.; Chae, S.Y.; Chae, W.B. Physiological traits associated with high temperature tolerance differ by fruit types and sizes in tomato (Solanum lycopersicum L.). Hortic. Environ. Biotechnol. 2020, 61, 837–847. [Google Scholar] [CrossRef]
- Ruggieri, V.; Calafiore, R.; Schettini, C.; Rigano, M.M.; Olivieri, F.; Frusciante, L.; Barone, A. Exploiting genetic and genomic resources to enhance heat-tolerance in tomatoes. Agronomy 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Kjaer, K.H.; Rosenqvist, E.; Yu, X.; Wu, Z.; Ottosen, C.-O. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J. Agron. Crop Sci. 2017, 203, 68–80. [Google Scholar] [CrossRef]
- Shamshiri, R.; Jones, J.; Thorp, K.; Ahmad, D.; Che Man, H.; Taheri, S. Review of optimum temperature, humidity, and vapour pressure deficit for microclimate evaluation and control in greenhouse cultivation of tomato: A review. Int. Agrophys. 2018, 32, 287–302. [Google Scholar] [CrossRef]
- Sharma, D.K.; Fernández, J.O.; Rosenqvist, E.; Ottosen, C.-O.; Andersen, S.B. Genotypic response of detached leaves versus intact plants for chlorophyll fluorescence parameters under high temperature stress in wheat. J. Plant Physiol. 2014, 171, 576–586. [Google Scholar] [CrossRef]
- Kalloo, G.; Chaurasia, S.N.S.; Singh, M. Stability analysis in tomato. Veg. Sci. 1998, 25, 81–84. Available online: https://worldveg.tind.io/record/10648 (accessed on 15 August 2020).
- Dorai, M.; Papadopoulos, A.P.; Gosselin, A. Influence of electric conductivity management on greenhouse tomato yield and fruit quality. Agronomie 2001, 21, 367–383. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Baki, A.A.; Stommel, R.R. Pollen viability and fruit set of tomato genotypes under optimum- and high-temperature regimes. HortScience 1995, 30, 115–117. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Craufurd, P.Q.; Summerfield, R.J. Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Ann. Bot. 1999, 84, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Djanaguirman, M.; Prasad, P.V.V.; Boyle, D.L.; Schapaugh, W.T. Soybean pollen anatomy, viability and pod set under high temperature stress. J. Agron. Crop Sci. 2013, 199, 171–177. [Google Scholar] [CrossRef]
- Alsamir, M.; Ahmad, N.; Arief, V.; Mahmood, T.; Trethowan, R. Phenotypic diversity and marker-trait association studies under heat stress in tomato (Solanum lycopersicum L.). Aust. J. Crop Sci. 2019, 13, 578–587. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Bheemanhalli, R.; Jagadish, S.V.K. Field crops and the fear of heat stress—Opportunities, challenges and future directions. Field Crop Res. 2017, 200, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.V.V.; Djanaguirman, M. Response of floret fertility and individual grain weight to high temperature stress: Sensitive, stages and thresholds for temperature and duration. Funt. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.V.V.; Djanaguirman, M.; Perumal, R.; Ciampitti, I.A. Impact of high temperature stress on floret fertility and individual grain weight of grain sorghum: Sensitive stages and thresholds for temperature and duration. Front. Plant. Sci. 2015, 6, 820. [Google Scholar] [CrossRef]
- Kanwar, M.S. Performance of tomato under greenhouse and open field conditions in the trans-Himalayan region of India. Adv. Horticult. Sci. 2011, 25, 65–68. Available online: https://0-www-jstor-org.brum.beds.ac.uk/stable/42882810 (accessed on 23 December 2020).
- Coste, S.; Baraloto, C.; Leroy, C.; Marcon, É.; Renaud, A.; Richardson, A.D.; Roggy, J.-C.; Schimann, H.; Uddling, J.; Hérault, B. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: A calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 2010, 67, 607. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maturo, T. A correlation analysis on chlorophyll content and SPAD value in tomato leaves. HortResearch 2017, 71, 37–42. Available online: http://0-doi-org.brum.beds.ac.uk/10.20776/S18808824-71-P37 (accessed on 22 December 2020).
- Ruiz-Espinoza, F.H.; Murillo-Amador, B.; García-Hernández, J.L.; Fenech-Larios, L.; Rueda-Puente, E.O.; Troyo-Diéguez, E.; Kaya, C.; Beltrán-Morales, A. Field evaluation of the relationship between chlorophyll content in basil leaves and a portable chlorophyll meter (SPAP-502) readings. J. Plant Nutr. 2010, 33, 423–438. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Guyer, L.; Hofstetter, S.S.; Christ, B.; Lira, B.S.; Rossi, M.; Hortensteiner, S. Different mechanisms are responsible for chlorophyll dephytylation during fruit ripening and leaf scenesence in tomato. Plant Physiol. 2014, 166, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, S.; Harvey, J.T.; Djidonou, D.; Leskovar, D.I. Exploring morpho-physiological variation for heat stress tolerance in tomato. Plants 2021, 10, 347. [Google Scholar] [CrossRef]
- Dane, F.; Hunter, A.; Chambliss, P.L. Fruit set, pollen fertility, and combining ability of selected tomato genotypes under high-temperature field conditions. J. Am. Soc. Hort. Sci. 1991, 116, 906–910. [Google Scholar] [CrossRef]
- AVRDC. 2017. Available online: https://avrdc.org/seed/improved-lines/processingdual-purpose-tomato/ (accessed on 19 August 2020).
- Solankey, S.S.; Akhtar, S.; Neha, P.; Kumari, M.; Kherwa, R. Screening and identification of heat tolerant tomato genotypes for Bihar. J. Pharm. Phytochem. 2018, 7, 97–100. [Google Scholar]
- Sangu, E.; Tibazarwa, F.I.; Nyomora, A.; Symonds, R.C. Expression of genes for the biosynthesis of compatible solutes during pollen development under heat stress in tomato (Solanum lycopersicum). J. Plant Physiol. 2015, 178, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Ampomah-Dwamena, C.; Sodedji, F.A.K.; Asante, I.K.; Danquah, E.Y. Accelerating breeding for heat tolerance in tomato (Solanum lycopersicum L.): An integrated approach. Agronomy 2019, 9, 720. [Google Scholar] [CrossRef] [Green Version]
- Cambodian Agricultural Research and Development Institute. Farmer Notes. 2007. Available online: https://www.cardi.org.kh/download.php?&file=a94.pdf (accessed on 8 January 2021).




| Source | df | Fruit | Biomass (g plant−1) | Chlorophyll Index (SPAD Reading) | ||||
|---|---|---|---|---|---|---|---|---|
| Length (cm) | Diameter (cm) | Setting (%) | Single Weight (g) | Yield (g plant−1) | ||||
| Genotypes (G) | 10 | ** | ** | ** | ** | ** | ** | * |
| Conditions (C) | 2 | ** | ** | ** | ** | ** | ** | ** |
| G X C | 20 | ** | ** | ** | ** | ** | ** | * |
| Error | 64 | |||||||
| Total | 98 | |||||||
| Genotypes | Length (mm) | Diameter (mm) | ||||
|---|---|---|---|---|---|---|
| OTFC | HTFC | HTGC | OTFC | HTFC | HTGC | |
| CLN3736D | 51.62 c ± 3.01 | 31.45 e ± 5.06 | 39.39 ef ± 1.28 | 53.21 bcd ± 3.70 | 34.50 g ± 2.22 | 47.08 cd ± 1.21 |
| CLN3078G | 61.49 b ± 3.72 | 37.30 de ± 8.36 | 59.91 ab ± 3.49 | 46.22 de ± 1.16 | 35.55 fg ± 2.96 | 44.45 de ± 1.18 |
| CLN3078C | 60.56 b ± 2.03 | 51.53 bc ± 1.78 | 53.65 bc ± 6.68 | 46.85 de ± 2.84 | 39.99 def ± 0.76 | 46.40 cd ± 1.97 |
| CLN3212C | 54.50 bc ± 2.36 | 46.15 cd ± 1.86 | 50.35 bcd ± 0.45 | 58.64 b ± 5.36 | 48.03 b ± 2.04 | 53.31 ab ± 2.82 |
| CLN3024A | 55.97 bc ± 3.55 | 42.72 cde ± 5.36 | 40.90 def ± 0.23 | 56.82 bc ± 3.48 | 46.48 bc ± 1.40 | 35.98 f ± 1.03 |
| CLN2498D | 54.51 bc ± 3.13 | 62.22 ab ± 3.88 | 56.54 abc ± 4.43 | 46.81 de ± 0.04 | 46.22 bc ± 3.47 | 48.39 bcd ± 1.08 |
| CLN3125L | 78.76 a ± 1.82 | 71.01 a ± 3.05 | 63.36 a ± 2.80 | 48.83 d ± 1.31 | 41.37 cde ± 2.16 | 39.79 ef ± 1.70 |
| CLN1621L | 40.78 e ± 1.40 | 37.57 de ± 1.23 | 38.20 f ± 1.51 | 40.79 e ± 2.20 | 37.06 efg ± 1.01 | 36.40 f ± 0.22 |
| CLN2026D | 60.06 b ± 4.83 | 51.85 bc ± 2.53 | 48.34 cde ± 3.59 | 50.44 cd ± 2.55 | 41.07 cde ± 1.61 | 43.73 de ± 3.37 |
| Neang Tamm | 43.28 de ± 2.04 | 45.74 cd ± 0.48 | 47.24 cdef ± 1.47 | 47.86 de ± 2.93 | 44.12 bcd ± 1.17 | 51.20 abc ± 3.12 |
| KK1 | 49.98 cd ± 2.20 | 43.05 cde ± 3.53 | 39.25 ef ± 3.98 | 65.97 a ± 4.20 | 53.90 a ± 3.85 | 55.07 a ± 1.82 |
| Mean (condition) | 55.59 | 47.33 | 48.83 | 51.06 | 42.57 | 45.62 |
| Genotypes | Fruit Setting (%) | Biomass (g plant−1) | ||||
|---|---|---|---|---|---|---|
| OTFC | HTFC | HTGC | OTFC | HTFC | HTGC | |
| CLN3736D | 62.59 ab ± 14.81 | 11.79 de ± 4.55 | 25.20 bcde ± 8.01 | 242.78 ab ± 18.21 | 188.89 ab ± 38.49 | 141.42 a ± 8.96 |
| CLN3078G | 41.07 ab ± 6.73 | 8.25 e ± 2.71 | 8.83 f ± 3.25 | 278.62 a ± 55.23 | 194.44 a ± 34.69 | 105.12 ab ± 3.88 |
| CLN3078C | 50.21 ab ± 13.48 | 28.64 abc ± 4.35 | 23.82 bcde ± 1.75 | 259.85 ab ± 75.67 | 188.89 ab ± 50.92 | 138.82 ab ± 38.76 |
| CLN3212C | 51.34 ab ± 7.88 | 34.50 ab ± 5.66 | 32.03 ab ± 1.36 | 167.65 abc ± 17.95 | 161.11 abcd ± 41.94 | 65.923 ab ± 4.27 |
| CLN3024A | 47.48 ab ± 6.17 | 28.64 abc ± 3.23 | 33.50 ab ± 2.23 | 155 bc ± 16.17 | 153.45 abcd ± 35 | 124.83 ab ± 8.40 |
| CLN2498D | 53.82 ab ± 4.51 | 12.07 de ± 2.31 | 13.73 ef ± 2.08 | 183.33 abc ± 16.67 | 168.03 abc ± 27.60 | 142.27 a ± 10.74 |
| CLN3125L | 39.70 abc ± 10.13 | 30.20 abc ± 5.75 | 22.52 cde ± 5.99 | 277.78 a ± 38.49 | 178.60 ab ± 9.91 | 113.35 ab ± 15.08 |
| CLN1621L | 43.09 abc ± 2.57 | 40.26 a ± 1.63 | 39.06 a ± 6.32 | 183.33 abc ± 16.67 | 100.35 bcd ± 5.25 | 55.423 b ± 12.39 |
| CLN2026D | 38.65 bc ± 3.41 | 18.16 cde ± 2.26 | 29.09 abcd ± 4.08 | 178.88 abc ± 46.23 | 155.56 abcd ± 19.25 | 82.253 ab ± 34.51 |
| Neang Tamm | 65.29 a ± 6.59 | 22.53 bcd ± 7.39 | 31.10 abcd ± 2.08 | 72.22 c ± 63.10 | 70.22 d ± 26.23 | 85.270 ab ± 76.58 |
| KK1 | 28.62 c ± 9.49 | 7.77 e ± 13.09 | 18.54 def ± 6.50 | 150.00 ab ± 16.67 | 83.13 cd ± 1.58 | 89.050 ab ± 3.12 |
| Mean (condition) | 38.71 | 22.09 | 36.20 | 205.97 | 149.33 | 103.98 |
| Genotype | Fruit Yield (g plant−1) | Mean | Fruit Yield Deviation from OTFC | |||||
|---|---|---|---|---|---|---|---|---|
| OTFC | HTFC | HTGC | HTFC | % Decrease | HTGC | % Decrease | ||
| CLN3736D | 1564.4 abc ± 432.84 | 22.06 f ± 5.56 | 72.33 f ± 13.14 | 552.93 | −1542.34 | −98.59 | −1492.07 | −95.38 |
| CLN3078G | 2027.0 ab ± 205.38 | 101.90 def ± 9.52 | 154.13 ef ± 42.63 | 761.01 | −1925.1 | −94.97 | −1872.87 | −92.40 |
| CLN3078C | 2080.6 a ± 373.10 | 295.91 cde ± 57.21 | 346.00 cde ± 191.76 | 907.50 | −1784.69 | −85.78 | −1734.6 | −83.37 |
| CLN3212C | 723.4 cd ± 35.01 | 454.94 bc ± 31.24 | 666.00 ab ± 88 | 614.78 | −268.46 | −37.11 | −57.4 | −7.93 |
| CLN3024A | 1580.9 ab ± 457.66 | 70.33 ef ± 3.33 | 190.13 ef ± 52.63 | 613.79 | −1510.57 | −95.55 | −1390.77 | −87.97 |
| CLN2498D | 1273.9 bc ± 341.99 | 310.66 cde ± 77.91 | 257.50 def ± 31.26 | 614.02 | −963.24 | −75.61 | −1016.4 | −79.79 |
| CLN3125L | 2179.7 a ± 129.30 | 227.29 cdef ± 15.57 | 174.50 ef ± 20.75 | 860.50 | −1952.41 | −89.57 | −2005.2 | −91.99 |
| CLN1621L | 1900.8 ab ± 6.34 | 586.99 ab ± 43.81 | 617.50 ab ± 89.75 | 1035.10 | −1313.81 | −69.12 | −1283.3 | −67.51 |
| CLN2026D | 2149.1 a ± 534.21 | 354.92 bcd ± 39.73 | 511.08 bcd ± 96.50 | 1005.03 | −1794.18 | −83.49 | −1638.02 | −76.22 |
| Neang Tamm | 640.7 d ± 67.78 | 322.09 cde ± 106. 31 | 599.00 abc ± 48.75 | 520.60 | −318.61 | −49.73 | −41.7 | −6.51 |
| KK1 | 1707.7 ab ± 100.60 | 795.01 a ± 239.15 | 818.33 a ± 91.73 | 1107.01 | −912.69 | −53.44 | −889.37 | −52.08 |
| Soil Properties | OTFC | HTFC | HTGC |
|---|---|---|---|
| Soil pH (H2O, 1:2.5) | 6.6 | 7.1 | 7.3 |
| Soil organic matter (Walkley & Black wet composition) | 0.76% | 1.01% | 1.01% |
| Total nitrogen (N) (Kjeldahl digestion) | 0.04% | 0.03% | 0.02% |
| Available phosphorus (P) (Olsen method) | 17.2 ppm | 32.7 ppm | 8.2 ppm |
| CEC (Ammonium acetate pH 7.0) | 11.8 cmolc/kg | 13.3 cmolc/kg | 21.9 cmolc/kg |
| Sand | 60.4 | 60.00% | 41.50% |
| Silt | 23.9 | 21.90% | 34.90% |
| Clay | 15.7 | 18.10% | 23.60% |
| Texture (Hydrometer method-USDA) | Sandy loam | Sandy Loam | Loam |
| Genotypes | Growth Habit | Heat Tolerance | Source |
|---|---|---|---|
| CLN3736D | Semi-determinate | Fair | WorldVeg |
| CLN3078C CLN3078G | Determinate Determinate | Moderate Good | WorldVeg WorldVeg |
| CLN3212C | Semi-determinate | Good | WorldVeg |
| CLN3024A | Determinate | Moderate | WorldVeg |
| CLN2898D | Semi-determinate | Moderate | WorldVeg |
| CLN3125L | Determinate | Moderate | WorldVeg |
| CLN1621L | Determinate | Good | WorldVeg |
| CLN2026D | Determinate | Good | WorldVeg |
| Neang Tamm | Determinate | Good | CARDI |
| KK1 | Determinate | Good | KVRS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ro, S.; Chea, L.; Ngoun, S.; Stewart, Z.P.; Roeurn, S.; Theam, P.; Lim, S.; Sor, R.; Kosal, M.; Roeun, M.; et al. Response of Tomato Genotypes under Different High Temperatures in Field and Greenhouse Conditions. Plants 2021, 10, 449. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030449
Ro S, Chea L, Ngoun S, Stewart ZP, Roeurn S, Theam P, Lim S, Sor R, Kosal M, Roeun M, et al. Response of Tomato Genotypes under Different High Temperatures in Field and Greenhouse Conditions. Plants. 2021; 10(3):449. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030449
Chicago/Turabian StyleRo, Sophoanrith, Leangsrun Chea, Sreymey Ngoun, Zachary P. Stewart, Siranet Roeurn, Penghieng Theam, Sathya Lim, Rathana Sor, Meas Kosal, Malean Roeun, and et al. 2021. "Response of Tomato Genotypes under Different High Temperatures in Field and Greenhouse Conditions" Plants 10, no. 3: 449. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030449