Then There Were Plenty-Ring Meristems Giving Rise to Many Stamen Whorls
Abstract
:1. Introduction
2. Regulation of Stem Cell Activity in the Floral Meristem
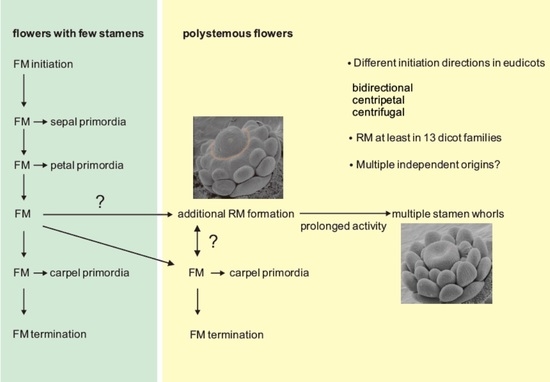
3. How to Generate Multiple Stamen Primordia
4. Ring Meristems in Eudicots
5. Are Ring Meristems in Dicots of Independent Origin?
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanderbali, A.S.; Berger, B.A.; Howarth, D.G.; Soltis, D.E.; Soltis, P.S. Evolution of floral diversity: Genomics, genes and gamma. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltis, P.S.; Brockington, S.F.; Yoo, M.-J.; Piedrahita, A.; Latvis, M.; Moore, M.J.; Chanderbali, A.S.; Soltis, D.E. Floral variation and floral genetics in basal angiosperms. Am. J. Bot. 2009, 96, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Endress, P.K. The early evolution of the angiosperm flower. Trends Ecol. Evol. 1987, 2, 300–304. [Google Scholar] [CrossRef]
- Denay, G.; Chahtane, H.; Tichtinsky, G.; Parcy, F. A flower is born: An update on Arabidopsis floral meristem formation. Curr. Opin. Plant Biol. 2017, 35, 15–22. [Google Scholar] [CrossRef]
- Chang, W.; Guo, Y.; Zhang, H.; Liu, X.; Guo, L. Same Actor in Different Stages: Genes in Shoot Apical Meristem Maintenance and Floral Meristem Determinacy in Arabidopsis. Front. Ecol. Evol. 2020, 8. [Google Scholar] [CrossRef] [Green Version]
- Furutani, M.; Nakano, Y.; Tasaka, M. MAB4-induced auxin sink generates local auxin gradients in Arabidopsis organ formation. Proc. Natl. Acad. Sci. USA 2014, 111, 1198–1203. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Ehlers, K. Arabidopsis flower development—Of protein complexes, targets, and transport. Protoplasma 2016, 253, 219–230. [Google Scholar] [CrossRef]
- Schrager-Lavelle, A.; Klein, H.; Fisher, A.; Bartlett, M. Grass flowers: An untapped resource for floral evo-devo. JNL Syst. Evol. 2017, 55, 525–541. [Google Scholar] [CrossRef] [Green Version]
- Theißen, G.; Melzer, R.; Rümpler, F. MADS-domain transcription factors and the floral quartet model of flower development: Linking plant development and evolution. Development 2016, 143, 3259–3271. [Google Scholar] [CrossRef] [Green Version]
- Kramer, E.M. Plus ça change, plus c’est la même chose: The developmental evolution of flowers. Curr. Top. Dev. Biol. 2019, 131, 211–238. [Google Scholar] [CrossRef]
- Smyth, D.R. Evolution and genetic control of the floral ground plan. New Phytol. 2018, 220, 70–86. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Xu, Y.; Ng, K.-H.; Ito, T. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 2009, 23, 1791–1804. [Google Scholar] [CrossRef] [Green Version]
- Emadzade, K.; Lehnebach, C.; Lockhart, P.; Hörandl, E. A molecular phylogeny, morphology and classification of genera of Ranunculeae (Ranunculaceae). Taxon 2010, 59, 809–828. [Google Scholar] [CrossRef]
- Rudall, P.J.; Sokoloff, D.D.; Remizowa, M.V.; Conran, J.G.; Davis, J.I.; Macfarlane, T.D.; Stevenson, D.W. Morphology of Hydatellaceae, an anomalous aquatic family recently recognized as an early-divergent angiosperm lineage. Am. J. Bot. 2007, 94, 1073–1092. [Google Scholar] [CrossRef] [Green Version]
- Shang, E.; Ito, T.; Sun, B. Control of floral stem cell activity in Arabidopsis. Plant Signal. Behav. 2019, 14, 1659706. [Google Scholar] [CrossRef]
- Long, J.A.; Moan, E.I.; Medford, J.I.; Barton, M.K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 1996, 379, 66–69. [Google Scholar] [CrossRef]
- Laux, T.; Mayer, K.F.; Berger, J.; Jurgens, G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 1996, 122, 87–96. [Google Scholar] [CrossRef]
- Clark, S.E.; Jacobsen, S.E.; Levin, J.Z.; Meyerowitz, E.M. The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in Arabidopsis. Development 1996, 122, 1567–1575. [Google Scholar] [CrossRef]
- Lenhard, M.; Bohnert, A.; Jürgens, G.; Laux, T. Termination of Stem Cell Maintenance in Arabidopsis Floral Meristems by Interactions between WUSCHEL and AGAMOUS. Cell 2001, 105, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Gallois, J.-L.; Nora, F.R.; Mizukami, Y.; Sablowski, R. WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev. 2004, 18, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, A.; Betsuyaku, S.; Osakabe, Y.; Mizuno, S.; Nagawa, S.; Stahl, Y.; Simon, R.; Yamaguchi-Shinozaki, K.; Fukuda, H.; Sawa, S. RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development 2010, 137, 3911–3920. [Google Scholar] [CrossRef] [Green Version]
- Brand, U.; Fletcher, J.C.; Hobe, M.; Meyerowitz, E.M.; Simon, R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 2000, 289, 617–619. [Google Scholar] [CrossRef]
- Fletcher, J.C.; Brand, U.; Running, M.P.; Simon, R.; Meyerowitz, E.M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 1999, 283, 1911–1914. [Google Scholar] [CrossRef]
- Lohmann, J.U.; Hong, R.L.; Hobe, M.; Busch, M.A.; Parcy, F.; Simon, R.; Weigel, D. A Molecular Link between Stem Cell Regulation and Floral Patterning in Arabidopsis. Cell 2001, 105, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Liu, X.; Yan, J.; Wang, W.; Yumul, R.E.; Kim, Y.J.; Dinh, T.T.; Liu, J.; Cui, X.; Zheng, B.; et al. ARGONAUTE10 and ARGONAUTE1 regulate the termination of floral stem cells through two microRNAs in Arabidopsis. PLoS Genet. 2011, 7, e1001358. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Looi, L.-S.; Guo, S.; He, Z.; Gan, E.-S.; Huang, J.; Xu, Y.; Wee, W.-Y.; Ito, T. Timing mechanism dependent on cell division is invoked by Polycomb eviction in plant stem cells. Science 2014, 343, 1248559. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, T.; Zheng, B.; Yumul, R.E.; Liu, X.; You, C.; Gao, Z.; Xiao, L.; Chen, X. APETALA2 antagonizes the transcriptional activity of AGAMOUS in regulating floral stem cells in Arabidopsis thaliana. New Phytol. 2017, 215, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 2004, 303, 2022–2025. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Kim, Y.; Dinh, T.T.; Chen, X. miR172 regulates stem cell fate and defines the inner boundary of APETALA3 and PISTILLATA expression domain in Arabidopsis floral meristems. Plant J. 2007, 51, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Würschum, T.; Groß-Hardt, R.; Laux, T. APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 2006, 18, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Prunet, N.; Morel, P.; Thierry, A.-M.; Eshed, Y.; Bowman, J.L.; Negrutiu, I.; Trehin, C. REBELOTE, SQUINT, and ULTRAPETALA1 function redundantly in the temporal regulation of floral meristem termination in Arabidopsis thaliana. Plant Cell 2008, 20, 901–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, A.T.; Stehling-Sun, S.; Offenburger, S.-L.; Lohmann, J.U. The bZIP Transcription Factor PERIANTHIA: A Multifunctional Hub for Meristem Control. Front. Plant Sci. 2011, 2, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carles, C.C.; Choffnes-Inada, D.; Reville, K.; Lertpiriyapong, K.; Fletcher, J.C. ULTRAPETALA1 encodes a SAND domain putative transcriptional regulator that controls shoot and floral meristem activity in Arabidopsis. Development 2005, 132, 897–911. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Prunet, N.; Gan, E.-S.; Wang, Y.; Stewart, D.; Wellmer, F.; Huang, J.; Yamaguchi, N.; Tatsumi, Y.; Kojima, M.; et al. SUPERMAN regulates floral whorl boundaries through control of auxin biosynthesis. EMBO J. 2018, 37. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Huang, J.; Xu, Y.; Tanoi, K.; Ito, T. Fine-tuning of auxin homeostasis governs the transition from floral stem cell maintenance to gynoecium formation. Nat. Commun. 2017, 8, 1125. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wang, R.; Zi, H.; Li, Y.; Cao, X.; Li, D.; Guo, L.; Tong, J.; Pan, Y.; Jiao, Y.; et al. AUXIN RESPONSE FACTOR3 Regulates Floral Meristem Determinacy by Repressing Cytokinin Biosynthesis and Signaling. Plant Cell 2018, 30, 324–346. [Google Scholar] [CrossRef] [Green Version]
- Galli, M.; Gallavotti, A. Expanding the Regulatory Network for Meristem Size in Plants. Trends Genet. 2016, 32, 372–383. [Google Scholar] [CrossRef]
- Thompson, B.E.; Bartling, L.; Whipple, C.; Hall, D.H.; Sakai, H.; Schmidt, R.; Hake, S. bearded-ear encodes a MADS box transcription factor critical for maize floral development. Plant Cell 2009, 21, 2578–2590. [Google Scholar] [CrossRef] [Green Version]
- Ohmori, S.; Kimizu, M.; Sugita, M.; Miyao, A.; Hirochika, H.; Uchida, E.; Nagato, Y.; Yoshida, H. MOSAIC FLORAL ORGANS1, an AGL6-like MADS box gene, regulates floral organ identity and meristem fate in rice. Plant Cell 2009, 21, 3008–3025. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, K.; Reisewitz, P.; Aoyama, T.; Friedrich, T.; Ando, S.; Sato, Y.; Tamada, Y.; Nishiyama, T.; Hiwatashi, Y.; Kurata, T.; et al. WOX13-like genes are required for reprogramming of leaf and protoplast cells into stem cells in the moss Physcomitrella patens. Development 2014, 141, 1660–1670. [Google Scholar] [CrossRef] [Green Version]
- Nardmann, J.; Werr, W. Symplesiomorphies in the WUSCHEL clade suggest that the last common ancestor of seed plants contained at least four independent stem cell niches. New Phytol. 2013, 199, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Sauquet, H.; von Balthazar, M.; Magallón, S.; Doyle, J.A.; Endress, P.K.; Bailes, E.J.; Barroso de Morais, E.; Bull-Hereñu, K.; Carrive, L.; Chartier, M.; et al. The ancestral flower of angiosperms and its early diversification. Nat. Commun. 2017, 8, 16047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endress, P.K. Evolutionary diversification of the flowers in angiosperms. Am. J. Bot. 2011, 98, 370–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, A.; Gleissberg, S.; Smyth, D.R. Floral and Vegetative Morphogenesis in California Poppy (Eschscholzia californica Cham.). Int. J. Plant Sci. 2005, 166, 537–555. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- François, L.; Verdenaud, M.; Fu, X.; Ruleman, D.; Dubois, A.; Vandenbussche, M.; Bendahmane, A.; Raymond, O.; Just, J.; Bendahmane, M. A miR172 target-deficient AP2-like gene correlates with the double flower phenotype in roses. Sci. Rep. 2018, 8, 12912. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Bachelier, J.B.; ZHANG, X.-H.; Ren, Y. Floral organogenesis in Dysosma versipellis (Berberidaceae) and its systematic implications. Botany 2016, 94, 359–368. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ren, Y.I.; Tian, X.H. Floral morphogenesis in Sinofranchetia (Lardizabalaceae) and its systematic significance. Bot. J. Linn. Soc. 2009, 160, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gong, J.; Zhao, L.; Che, X.; Li, H.; Ren, Y. Flower morphology and development of the monotypic Chinese genus Anemoclema (Ranunculaceae). Plant Syst. Evol. 2016, 302, 683–690. [Google Scholar] [CrossRef]
- Tucker, S.C. Floral ontogeny in Swartzia (Leguminosae: Papilionoideae: Swartzieae): Distribution and role of the ring meristem. Am. J. Bot. 2003, 90, 1271–1292. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, L.; Ren, Y. Development of flowers and inflorescences of Circaeaster (Circaeasteraceae, Ranunculales). Plant Syst. Evol. 2005, 256, 89–96. [Google Scholar] [CrossRef]
- Schuchovski, C.; Meulia, T.; Sant’Anna-Santos, B.F.; Fresnedo-Ramírez, J. Inflorescence Development and Floral Organogenesis in Taraxacum kok-saghyz. Plants 2020, 9, 1258. [Google Scholar] [CrossRef]
- Ren, Y.; Li, H.-F.; Zhao, L.; Endress, P.K. Floral morphogenesis in Euptelea (Eupteleaceae, Ranunculales). Ann. Bot. 2007, 100, 185–193. [Google Scholar] [CrossRef]
- Endress, P.K.; Matthews, M.L. Progress and problems in the assessment of flower morphology in higher-level systematics. Plant Syst. Evol. 2012, 298, 257–276. [Google Scholar] [CrossRef] [Green Version]
- Endress, P.K. Flower Structure and Trends of Evolution in Eudicots and Their Major Subclades 1. Ann. Mo. Bot. Gard. 2010, 97, 541–583. [Google Scholar] [CrossRef]
- Endress, P.K. Angiosperm Floral Evolution: Morphological Developmental Framework. In Advances in Botanical Research: Incorporating Advances in Plant Pathology; Callow, J.A., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2006; pp. 1–61. ISBN 9780120059447. [Google Scholar]
- Endress, P.K. The families and Genera of Vascular Plants II. Flower. Plants 1993, 372, 599–602. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Becker, A. Then There Were Plenty-Ring Meristems Giving Rise to Many Stamen Whorls. Plants 2021, 10, 1140. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10061140
Kong D, Becker A. Then There Were Plenty-Ring Meristems Giving Rise to Many Stamen Whorls. Plants. 2021; 10(6):1140. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10061140
Chicago/Turabian StyleKong, Doudou, and Annette Becker. 2021. "Then There Were Plenty-Ring Meristems Giving Rise to Many Stamen Whorls" Plants 10, no. 6: 1140. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10061140