Ethephon-Induced Ethylene Enhances Starch Degradation and Sucrose Transport with an Interactive Abscisic Acid-Mediated Manner in Mature Leaves of Oilseed rape (Brassica napus L.)
Abstract
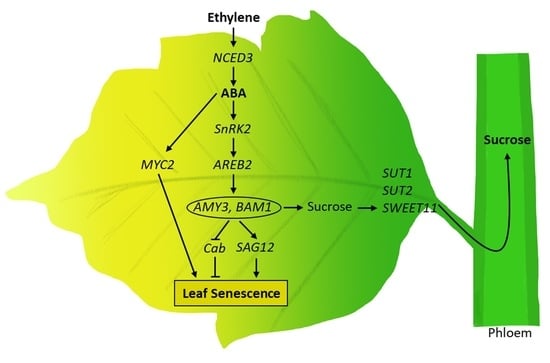
:1. Introduction
2. Material and Methods
2.1. Plant Culture and Experimental Procedure
2.2. Determination of Total Chlorophyll Content
2.3. Collection of Phloem Exudates and Determination of Soluble Sugars and Starch
2.4. Determination of Phytohormones
2.5. RNA Extraction and Quantitative Real-timePCR
2.6. Statistical Analysis
3. Results
3.1. Biomass, Chlorophyll Content, and Cab and SAG12 Gene Expression in Mature Leaves
3.2. Endogenous Hormonal Status and ABA Synthesis and Signaling-Related Gene Expression
3.3. Carbohydrate Status and Starch Degradation-related Gene Expression in Mature Leaves
3.4. Phloem Sucrose Loading and Sucrose Transport Gene Expression in Mature Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Avice, J.C.; Etienne, P. Leaf senescence and nitrogen remobilization efficiency in oilseed rape (Brassica napus L.). J. Exp. Bot. 2014, 65, 3813–3824. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Orsel, M. Leaf nitrogen remobilization for plant development and grain filling. Plant Biol. 2008, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Lo, S.F.; Ho, T.H.D. Source-sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, B.R.; Islam, M.T.; La, V.H.; Park, S.H.; Bae, D.W.; Kim, T.H. Cultivar variation in hormone- and sugar-response reveals abscisic acid responsive sucrose phloem loading at the early regenerative stage is a significant determinant of seed yield in Brassica napus. Environ. Exp. Bot. 2020, 169, 103917. [Google Scholar] [CrossRef]
- Tilsner, J.; Kassner, N.; Struck, C.; Lohaus, G. Amino acid contents and transport in oilseed rape (Brassica napus L.) under different nitrogen conditions. Planta 2005, 221, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Gironde, A.; Etienne, P.; Trouverie, J.; Bouchereau, A.; Le Caherec, F.; Leport, L.; Orsel, M.; Niogret, M.F.; Nesi, N.; Carole, D.; et al. The contrasting N management of two oilseed rape genotypes reveals the mechanisms of proteolysis associated with leaf N remobilization and the respective contributions of leaves and stems to N storage and remobilization during seed filling. BMC Plant Biol. 2015, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Lee, B.R.; Park, S.H.; Zaman, R.; Bae, D.W.; Kim, T.H. Evidence of salicylic acid-mediated protein degradation and amino acid transport in mature leaves of Brassica napus. J. Plant Growth Regul. 2015, 34, 684–689. [Google Scholar] [CrossRef]
- Poret, M.; Chandrasekar, B.; van der Hoorn, R.A.L.; Déchaumet, S.; Bouchereau, A.; Kim, T.H.; Lee, B.R.; Macquart, F.; Hara-Nishimura, I.; Avice, J.C. A genotypic comparison reveals that the improvement in nitrogen remobilization efficiency in oilseed rape leaves is related to specific patterns of senescence-associated protease activities and phytohormones. Front. Plant Sci. 2019, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Jianjun, C.; Richard, J.H.; Russell, D.C. Ethephon suppresses flowering and improves the aesthetic value of purple passion plant (Gynura aurantiaca). J. Environ. Hort. 2002, 20, 228–231. [Google Scholar]
- Jiang, Y.; Liang, G.; Yang, S.; Yu, D. Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid– and auxin-mediated sSignaling in jasmonic acid–induced leaf senescence. Plant Cell 2014, 26, 230–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, T.; John, A.C.; Subramanian, J.; Peter, K.P. Ethephon-induced abscission of “Redhaven” peach. Am. J. Plant Sci. 2012, 3, 295–301. [Google Scholar]
- Schaller, G.E. Ethylene and the regulation of plant development. BMC Biol. 2012, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grbic, V.; Bleecker, A.B. Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J. 1995, 8, 595–602. [Google Scholar] [CrossRef]
- John, I.; Drake, R.; Farell, A.; Cooper, W.; Lee, P.; Horton, P.; Grierson, D. Delayed leaf senescence in ethylene-deficient ACC oxidase antisense tomato plants: Molecular and physiological analysis. Plant J. 1995, 7, 483–490. [Google Scholar] [CrossRef]
- Koyama, T. The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Front. Plant Sci. 2014, 5, 650. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Chaffin, G.S.; Eason, J.R.; Clark, D.G. Ethylene sensitivity regulates proteolytic activity and cysteine protease gene expression in petunia corollas. J. Exp. Bot. 2005, 56, 2733–2744. [Google Scholar] [CrossRef]
- Chapin, L.J.; Jones, M.L. Ethylene regulates phosphorus remobilization and expression of a phosphate transporter (PhPT1) during petunia corolla senescence. J. Exp. Bot. 2009, 60, 2179–2190. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A. The influence of exogenous ethylene on growth and photosynthesis of mustard (Brassica juncea) following defoliation. Sci. Hortic. 2005, 105, 499–505. [Google Scholar] [CrossRef]
- Khan, N.A.; Mir, M.R.; Nazar, R.; Singh, S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biol. 2008, 10, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Hendgen, M.; Gunther, S.; Schubert, S.; Lohnertz, O. Ethephon activates the transcription of senescence-associated genes and nitrogen mobilization in grapevine leaves (Vitis vinifera cv. Riesling). Plants 2021, 10, 333. [Google Scholar] [CrossRef]
- Kasai, M. Regulation of leaf photosynthetic rate correlating with leaf carbohydrate status and activation state of Rubisco under a variety of photosynthetic source/sink balances. Physiol. Plant 2008, 134, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source-sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef] [Green Version]
- Coruzzi, G.M.; Zhou, L. Carbon and nitrogen sensing and signaling in plants: Emerging ‘matrix effects’. Curr. Opin. Plant Biol. 2001, 4, 247–253. [Google Scholar] [CrossRef]
- Gibson, S.I. Sugars and phytohormone response pathway: Navigating a signaling network. J. Exp. Bot. 2004, 55, 253–264. [Google Scholar] [CrossRef]
- Ljung, K.; Nemhauser, J.L.; Perata, P. New mechanistic links between sugar and hormone signaling networks. Curr. Opin. Plant Biol. 2015, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Roitsch, T. Metabolic regulation of leaf senescence: Interaction of sugar signaling with biotic and abiotic stress responses. Plant Biol. 2008, 10, 50–62. [Google Scholar] [CrossRef] [PubMed]
- La, V.H.; Lee, B.R.; Islam, M.T.; Park, S.H.; Lee, H.; Bae, D.W.; Kim, T.H. Antagonistic shifting from abscisic acid- to salicylic acid-mediated sucrose accumulation contributes to drought tolerance in Brassica napus. Environ. Exp. Bot. 2019, 162, 38–47. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.R.; Jin, Y.L.; Avice, J.C.; Cliquet, J.B.; Ourry, A.; Kim, T.H. Increased proline loading to phloem and its effects on nitrogen uptake and assimilation in water stressed white clover (Trifolium repens). New Phytol. 2009, 182, 654–663. [Google Scholar] [CrossRef]
- Baxter, C.J.; Foyer, C.H.; Turner, J.; Rolfe, S.A.; Quick, W.P. Elevated sucrose phosphate synthase activity in transgenic tobacco sustains photosynthesis in older leaves and alters development. J. Exp. Bot. 2003, 54, 1813–1820. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.Q.; Welti, R.; Wang, W.M. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- De la Torre, F.; del Carmen Rodríguez-Gacio, M.; Matilla, A.J. How ethylene works in the reproductive organs of higher plants. Plant Signal Behav. 2006, 1, 231–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopher, B.C.; William, B.M. Residual effects of ethylene on Tulip growth and flowering. Hort. Sci. 2010, 45, 1164–1166. [Google Scholar]
- Jibran, R.; Hunter, D.A.; Dijkwel, P.P. Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol. Biol. 2013, 82, 547–561. [Google Scholar] [CrossRef]
- Özgen, M.; Park, S.; Palta, J.P. Mitigation of ethylene-promoted leaf senescence by a natural lipid, lysophosphatidylethanolamine. Hortscience 2005, 40, 1166–1167. [Google Scholar] [CrossRef] [Green Version]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matile, P.; Hörtensteiner, S.; Thomas, H.; Kräutler, B. Chlorophyll breakdown in senescent leaves. Plant Physiol. 1996, 112, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan-Wollaston, V.; Ainsworth, C. Leaf senescence in Brassica napus: Cloning of senescence related genes by subtractive hybridisation. Plant Mol. Biol. 1997, 33, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [Green Version]
- Qiu, K.; Li, Z.; Yang, Z.; Chen, J.; Wu, S.; Zhu, X.; Gao, S.; Gao, J.; Ren, G.; Kual, B.; et al. EIN3 and ORE1 accelerate degreening during ethylene-mediated leaf senescence by directly activating chlorophyll catabolic genes in Arabidopsis. PloS Genet. 2015, 11, e1005399. [Google Scholar] [CrossRef]
- Jing, H.C.; Schippers, J.H.M.; Hille, J.; Dijkwel, P.P. Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis. J. Exp. Bot. 2005, 56, 2915–2923. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Di, C.; Zhang, Q.; Zhang, K.; Wang, C.; You, Q.; Yan, H.; Dai, S.Y.; Yan, J.S.; et al. JAZ7 negatively regulates dark-induced leaf senescence in Arabidopsis. J. Exp. Bot. 2016, 67, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Worley, E.; Udvardi, M. A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell 2014, 26, 4862–4874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Chen, J.; Xie, Z.; Gao, J.; Ren, G.; Gao, S.; Zhou, X.; Kuai, B. Jasmonic acid promotes degreening via MYC2/3/4- and AAC019/055/072-mediated regulation of major chlorophyll catabolic genes. Plant J. 2015, 84, 97–610. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gan, S. Leaf senescence: Signals, execution, and regulation. Curr. Top. Dev. Biol. 2005, 71, 83–112. [Google Scholar] [PubMed]
- Kusaba, M.; Tanaka, A.; Tanaka, R. Stay-green plants: What do they tell us about the molecular mechanism of leaf senescence. Photosynth. Res. 2013, 117, 221–234. [Google Scholar] [CrossRef]
- Lee, B.R.; Zaman, R.; Avice, J.C.; Ourry, A.; Kim, T.H. Sulfur use efficiency is a significant determinant of drought stress tolerance in relation to photosynthetic activity in Brassica napus cultivars. Front. Plant Sci. 2016, 7, 459. [Google Scholar] [CrossRef] [Green Version]
- Benschop, J.J.; Jackson, M.B.; Gühl, K.; Vreeburg, R.A.M.; Croker, S.J.; Peeters, A.J.; Voesenek, L.A. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J. 2005, 44, 756–768. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, L.; Yu, Y.; Quan, R.; Zhang, Z.; Zhang, H.; Huang, R. Ethylene response factor AtERF11 that is transcriptionally modulated by bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J. 2011, 68, 88–99. [Google Scholar] [CrossRef]
- Frankowski, K.; Wilmowicz, E.; Kucko, A.; Kesy, J.; Swiezawska, B.; Kopcewicz, J. Ethylene, auxin, and abscisic acid interactions in the control of photoperiodic flower induction in Pharbitis Nil. Biol. Plant. 2014, 58, 305–310. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water-stress during grain filling. Plant Cell Environ. 2003, 26, 1621–1631. [Google Scholar] [CrossRef]
- He, P.; Osaki, M.; Takebe, M.; Shinano, T.; Wasaki, J. Endogenous hormones and expression of senescence-related genes in different senescent types of maize. J. Exp. Bot. 2005, 56, 1117–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/AFB transcription factors function predominantly in gene expression downstream of SnRK2 kinase in abscisic acid signaling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Gao, J.; Zhu, X.; Song, Y.; Li, Z.; Ren, G.; Zhou, X.; Kuai, B. ABF2 ABF3 and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll catabolic genes and senescence-associated genes in Arabidopsis. Mol. Plant 2016, 9, 1272–1285. [Google Scholar] [CrossRef] [Green Version]
- Radchuk, R.; Emery, R.J.; Weier, D.; Vigeolas, H.; Geigenberger, P.; Lumn, J.E.; Feil, R.; Weschke, W.; Weber, H. Sucrose non-fermenting kinase 1 (SnRK1) coordinates metabolic and hormonal signals during pea cotyledon growth and differentiation. Plant J. 2010, 61, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Manalansan, B.; Rauniyar, N.; Munemasa, S.; Booker, M.A.; Brandt, B.; Waadt, C.; Nusinow, D.A.; Kay, S.A.; Kunz, H.H.; et al. Identification of open stomata1-interacting proteins reveals interactions with sucrose non-fermenting1-related protein kinases2 and with type 2A protein phosphatases that function in abscisic acid responses. Plant Physiol. 2015, 169, 760–779. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeihofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Xu, X.; Crosley, R.A.; Greenwalt, S.A.; Sun, Y.; Blakeslee, B.; Wang, L.; Ni, W.; Sopko, M.S.; Yao, C.; et al. The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol. 2010, 153, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Liu, Y.J.; Hu, D.G.; Hao, Y.J. Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol. 2017, 174, 2348–2362. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.S.; Zhang, S.L.; Wang, J.H. Sugar metabolism and its regulation in post-harvest ripening kiwifruit. J. Plant Physiol. Mol. Biol. 2004, 30, 23–26. [Google Scholar]
- Kępczyńska, E.; Zielińska, S. The role of endogenous ethylene in carbohydrate metabolism of Medicago sativa L. somatic embryos in relation to their regenerative ability. J. Plant Growth Regul. 2013, 32, 191–199. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-R.; Zaman, R.; La, V.H.; Bae, D.-W.; Kim, T.-H. Ethephon-Induced Ethylene Enhances Starch Degradation and Sucrose Transport with an Interactive Abscisic Acid-Mediated Manner in Mature Leaves of Oilseed rape (Brassica napus L.). Plants 2021, 10, 1670. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081670
Lee B-R, Zaman R, La VH, Bae D-W, Kim T-H. Ethephon-Induced Ethylene Enhances Starch Degradation and Sucrose Transport with an Interactive Abscisic Acid-Mediated Manner in Mature Leaves of Oilseed rape (Brassica napus L.). Plants. 2021; 10(8):1670. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081670
Chicago/Turabian StyleLee, Bok-Rye, Rashed Zaman, Van Hien La, Dong-Won Bae, and Tae-Hwan Kim. 2021. "Ethephon-Induced Ethylene Enhances Starch Degradation and Sucrose Transport with an Interactive Abscisic Acid-Mediated Manner in Mature Leaves of Oilseed rape (Brassica napus L.)" Plants 10, no. 8: 1670. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081670