Pilot Study: Does Contamination with Enniatin B and Beauvericin Affect the Antioxidant Capacity of Cereals Commonly Used in Animal Feeding?
Abstract
:1. Introduction
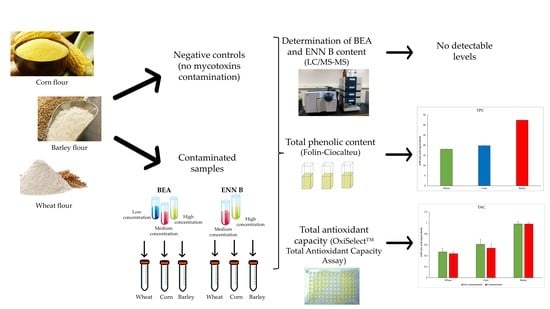
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Determination of Mycotoxins
3.3. Total Phenolic Content Assay
3.4. Extraction Procedure for Total Antioxidant Capacity Assay
3.4.1. Sample Treatment
Negative Controls
Contaminated Samples
3.5. Total Antioxidant Capacity (TAC) Assay
3.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organisation). World Agriculture: Towards 2015/2030: Summary Report; FAO: Rome, Italy, 2002. [Google Scholar]
- Hübner, F.; Arendt, E.K. Germination of cereal grains as a way to improve the nutritional value: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 853–861. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic acid profiles and antioxidant activity of major cereal crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- McKevith, B. Nutritional aspects of cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Juan, C.; Mañes, J.; Raiola, A.; Ritieni, A. Evaluation of beauvericin and enniatins in Italian cereal products and multicereal food by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem. 2013, 140, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. Structure of beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969, 49, 4255–4258. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Macáková, P.; Juan-García, A.; Font, G. Cytotoxic effects of mycotoxin combinations in mammalian kidney cells. Food Chem. Toxicol. 2011, 49, 2718e2724. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Juan-García, A.; Font, G.; Ruiz, M.J. Beauvericin-induced cytotoxicity via ROS production and mitochondrial damage in caco-2 cells. Toxicol. Lett. 2013, 222, 204e201. [Google Scholar] [CrossRef]
- Mallebrera, B.; Maietti, A.; Tedeschi, P.; Font, G.; Ruiz, M.J.; Brandolini, V. Antioxidant capacity of trans-resveratrol dietary supplements alone or combined with the mycotoxin beauvericin. Food Chem. Toxicol. 2017, 105, 315–318. [Google Scholar] [CrossRef]
- Calò, L.; Fornelli, F.; Ramires, R.; Nenna, S.; Tursi, A.; Caiaffa, M.F.; Macchia, L. Cytotoxic effects of the mycotoxin beauvericin to human cell lines of myeloid origin. Pharmacol. Res. 2004, 49, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Paciolla, C.; Dipierro, N.; Mulè, G.; Logrieco, A.; Dipierro, S. The mycotoxins beauvericin and T-2 induce cell death and alteration to the ascorbate metabolism in tomato protoplasts. Physiol. Mol. Plant. Pathol. 2004, 65, 49–56. [Google Scholar] [CrossRef]
- Ferrer, E.; Juan-Garcia, A.; Font, G.; Ruiz, M.J. Reactive oxygen species induced by beauvericin, patulin and zearalenone in CHO-K1 cells. Toxicol. Vitr. 2009, 23, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Mallebrera, B.; Brandolini, V.; Font, G.; Ruiz, M.J. Cytoprotective effect of resveratrol diastereomers in CHO-K1 cells exposed to beauvericin. Food Chem. Toxicol. 2015, 80, 319e327. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Dvořáčková, M.; Kašparovský, T. Feedborne mycotoxins beauvericin and enniatins and livestock animals. Toxins 2021, 13, 32. [Google Scholar] [CrossRef]
- Ivanova, L.; Egge-Jacobsen, W.M.; Solhaug, A.; Thoen, E.; Fæste, C.K. Lysosomes as a possible target of enniatin B-induced toxicity in Caco-2 cells. Chem. Res. Toxicol. 2012, 25, 1662–1674. [Google Scholar] [CrossRef] [PubMed]
- Dornetshuber, R.; Heffeter, P.; Lemmens-Gruber, R.; Elbling, L.; Marko, D.; Micksche, M.; Berger, W. Oxidative stress and DNA interactions are not involved in Enniatin- and Beauvericin mediated apoptosis induction. Mol. Nutr. Food Res. 2009, 53, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Svingen, T.; Lund Hansen, N.; Taxvig, C.; Vinggaard, A.M.; Jensen, U.; Have Rasmussen, P. Enniatin B and beauvericin are common in Danish cereals and show high hepatotoxicity on a high-content imaging platform. Environ. Toxicol. 2017, 32, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.F.; Girgin, G.; Baydar, T.; Krska, R.; Sulyok, M. Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS. J. Sci. Food Agric. 2017, 97, 4419–4428. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.; Grignon, G.; Vitry, C.; Kashefifard, K.; Valade, R. Fusarium species and enniatin mycotoxins in wheat, durum wheat, triticale and barley harvested in France. Mycotoxin Res. 2019, 35, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Rodríguez-Estévez, V.; Arenas-Fernández, P.; García-Campaña, A.M.; Gámiz-Gracia, L. Occurrence of mycotoxins in Swine feeding from Spain. Toxins 2019, 11, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, B.; Rainer, V.; Sulyok, M.; Haltrich, D.; Schatzmayr, G.; Mayer, E. Twenty-eight fungal secondary metabolites detected in pig feed samples: Their occurrence, relevance and cytotoxic effects in vitro. Toxins 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Serafini, M.; Bellocco, R.; Wolk, A.; Ekstrom, A.M. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology 2002, 123, 985–991. [Google Scholar] [CrossRef]
- Cecchini, S.; Fazio, F. Assessment of Total Antioxidant capacity in serum of heathy and stressed hens. Animals 2020, 10, 2019. [Google Scholar] [CrossRef]
- European Commission 2002/32/EC. Directive of The European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed 2002/32/EC. Off. J. Eur. Communities 2002, 140, 12. [Google Scholar]
- Perez-Jimenez, J.; Saura-Calixto, F. Literature data may underestimate the actual antioxidant capacityy of cereals. J. Agric. Food Chem. 2005, 53, 5036–5040. [Google Scholar] [CrossRef] [Green Version]
- Durazzo, A.; Casale, G.; Melini, V.; Maiani, G.; Acquistucci, R. Total polyphenol content and antioxidant properties of Solina (Triticum aestivum L.) and their derived products. Ital. J. Food Sci. 2016, 28, 221–229. [Google Scholar]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Giromini, C.; Tretola, M.; Baldi, A.; Ottoboni, M.; Rebucci, R.; Manoni, M.; Di Lorenzo, C.; Pinotti, L. Total phenolic content and antioxidant capacity of former food products intended as alternative feed ingredients. Ital. J. Anim. Sci. 2020, 19, 1387–1392. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Khadka, D.; Timilsina, R. Total phenolic contents and antioxidant activity profile of selected cereal sprouts and grasses. Int. J. Food Prop. 2019, 22, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef] [Green Version]
- Yamanouchi, K.; Sakamoto, Y.; Tsujiguchi, T. Contemporary traditional vegetables in Japan: Physiological function of buckwheat sprouts. J. Nutr. Food Sci. 2018, 8, 1000712. [Google Scholar] [CrossRef]
- Seo, Y.W.; Bu, S.Y.; Jeon, W.B.; Kim, D.S. Antioxidant activity and total phenolic compounds in grain extracts of wheat, barley, and oat. Korean J. Crop. Sci. 2002, 47, 102–107. [Google Scholar]
- Fogarasi, A.L.; Kun, S.; Tankó, G.; Stefanovits-Bányai, E.; Hegyesné-Vecseri, B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015, 167, 1–6. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidant potential of barley as affected by alkaline hydrolysis and release of insoluble-bound phenolics. Food Chem. 2009, 117, 615–620. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [Green Version]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Frangiamone, M.; Manyes, L. Transcriptional changes after enniatins A, A1, B and B1 ingestion in rat stomach, liver, kidney and lower intestine. Foods 2021, 10, 1630. [Google Scholar] [CrossRef]
- Vikas, V.K.; Tomar, S.M.S.; Sivasamy, M.; Kumar, J.; Jayaprakash, P.; Kumar, P.; Peter, J.; Nisha, R.; Punniakotti, E. Hybrid necrosis in wheat: Evolutionary significance or potential barrier for gene flow? Euphytica 2013, 194, 261–275. [Google Scholar] [CrossRef]
- Fiers, M.; Lognay, G.; Fauconnier, M.L.; Jijakli, M.H. Volatile compound-mediated interactions between barley and pathogenic fungi in the soil. PLoS ONE 2013, 8, e66805. [Google Scholar] [CrossRef] [Green Version]
- Dagnac, T.; Latorre, A.; Fernandez Lorenzo, B.; Llompart, M. Validation and application of a liquid chromatography-tandem mass spectrometry based method for the assessment of the co-occurrence of mycotoxins in maize silages from dairy farms in NW Spain. Food Addit. Contam. Part. A 2016, 33, 1850–1863. [Google Scholar] [CrossRef]
- Decleer, M.; Rajkovic, A.; Sas, B.; Madder, A.; De Saeger, S. Development and validation of ultra-high-performance liquid chromatography-tandem mass spectrometry methods for the simultaneous determination of beauvericin, enniatins (A, A1, B, B1) and cereulide in maize, wheat, pasta and rice. J. Chromatogr. A 2016, 1472, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Jestoi, M.; Rokka, M.; Järvenpää, E.; Peltonen, K. Determination of Fusarium mycotoxins beauvericin and enniatins (A, A1, B, B1) in eggs of laying hens using liquid chromatography-tandem mass spectrometry (LC–MS/MS). Food Chem. 2009, 115, 1120–1127. [Google Scholar] [CrossRef]
- Piatkowska, M.; Sulyok, M.; Pietruszka, K.; Panasiuk, Ł. Pilot study for the presence of fungal metabolites in sheep milk from first spring milking. J. Vet. Res. 2018, 62, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Meca, G.; Juan, C.; Mañes, J. Biosynthesis of beauvericin and enniatins in vitro by wheat Fusarium species and natural grain contamination in an area of central Italy. Food Microbiol. 2015, 46, 618–626. [Google Scholar] [CrossRef]
- COFRAC (French Committee for Accreditation). Technical Guide to Accreditation: Mycotoxins and Phycotoxins in Foodstuffs Intended for Humans or Animals. 2017. Available online: https://www.cofrac.fr/documentation/LAB-GTA-21 (accessed on 27 August 2021).
- Attard, E. A rapid microtiter plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Pozzo, L.; Salamano, G.; Mellia, E.; Gennero, M.; Doglione, L.; Cavallarin, L.; Tarantola, M.; Forneris, G.; Schiavone, A. Feeding a diet contaminated with ochratoxin A for chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg). 1. Effects on growth and slaughter performance, haematological and serum traits. J. Anim. Physiol. Anim. Nutr. 2013, 97, 13–22. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, V.; Salvatori, G.; Pastorelli, G. Pilot Study: Does Contamination with Enniatin B and Beauvericin Affect the Antioxidant Capacity of Cereals Commonly Used in Animal Feeding? Plants 2021, 10, 1835. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10091835
Serra V, Salvatori G, Pastorelli G. Pilot Study: Does Contamination with Enniatin B and Beauvericin Affect the Antioxidant Capacity of Cereals Commonly Used in Animal Feeding? Plants. 2021; 10(9):1835. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10091835
Chicago/Turabian StyleSerra, Valentina, Giancarlo Salvatori, and Grazia Pastorelli. 2021. "Pilot Study: Does Contamination with Enniatin B and Beauvericin Affect the Antioxidant Capacity of Cereals Commonly Used in Animal Feeding?" Plants 10, no. 9: 1835. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10091835