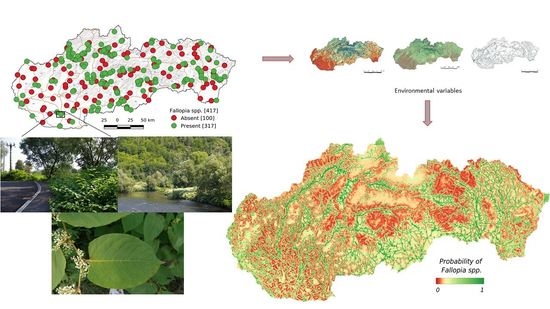
The Prediction of Distribution of the Invasive Fallopia Taxa in Slovakia
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Distance from Transport Lines
3.2. Soil Type
3.3. Distance from Water Bodies
4. Materials and Methods
4.1. Study Area
4.2. Fallopia Occurrence Data
4.3. Environmental Data
4.4. Ensemble Distribution Model Development and Evaluation
4.5. Logistic Regression
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vitousek, P.; Mooney, H.A.; Lubchenco, J.; Mellilo, J.M. Human Domination of Earth. Science 1997, 227, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Shea, K.; Chesson, P. Community Ecology Theory as a Framework for Biological Invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Genovesi, P.; Shine, C.; Europe, C. European Strategy on Invasive Alien Species: Convention on the Conservation of European Wildlife and Habitats (Bern Convention); Council of Europe: Strasbourg, France, 2004; ISBN 978-92-871-5488-0.
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Wong, E.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and Environmental Threats of Alien Plant, Animal, and Microbe Invasions. Agric. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive Species, Ecosystem Services and Human Well-Being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Kueffer, C. Plant Invasions in the Anthropocene. Science 2017, 358, 724–725. [Google Scholar] [CrossRef]
- Jones, B.A.; McDermott, S.M. Health Impacts of Invasive Species through an Altered Natural Environment: Assessing Air Pollution Sinks as a Causal Pathway. Environ. Resour. Econ. 2018, 71, 23–43. [Google Scholar] [CrossRef]
- Bartz, R.; Kowarik, I. Assessing the Environmental Impacts of Invasive Alien Plants: A Review of Assessment Approaches. NeoBiota 2019, 43, 69. [Google Scholar] [CrossRef] [Green Version]
- Pyšek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. SSRN 2010, 35, 25–55. [Google Scholar] [CrossRef] [Green Version]
- Stone, C.M.; Witt, A.B.; Walsh, G.C.; Foster, W.A.; Murphy, S.T. Would the Control of Invasive Alien Plants Reduce Malaria Transmission? A Review. Parasites Vectors 2018, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.A. Tree Shade, Temperature, and Human Health: Evidence from Invasive Species-Induced Deforestation. Ecol. Econ. 2019, 156, 12–23. [Google Scholar] [CrossRef]
- Gerber, E.; Krebs, C.; Murrell, C.; Moretti, M.; Rocklin, R.; Schaffner, U. Exotic Invasive Knotweeds (Fallopia Spp.) Negatively Affect Native Plant and Invertebrate Assemblages in European Riparian Habitats. Biol. Conserv. 2008, 141, 646–654. [Google Scholar] [CrossRef]
- Lodge, D.M. Biological Invasions: Lessons for Ecology. Trends Ecol. Evol. 1993, 8, 133–137. [Google Scholar] [CrossRef]
- Turlings, L. Invasive Plants and Animals: Is There a Way Out? Invasive plants and animals: Is there a way out? In Proceedings of the Conference on Alien Invasive Species, Leiden, The Netherlands, 26 September 2000; pp. 10–18. [Google Scholar]
- Mandák, B.; Pyšek, P.; Bímová, K. History of the Invasion and Distribution of Reynoutria Taxa in the Czech Republic: A Hybrid Spreading Faster than Its Parents. Preslia 2004, 76, 15–64. [Google Scholar]
- Beerling, D.J.; Bailey, J.P.; Conolly, A.P. Fallopia Japonica (Houtt.) Ronse Decraene (Reynoutria Japonica Houtt.; Polygonum Cuspidatum Sieb. & Zucc.). J. Ecol. 1994, 82, 959–979. [Google Scholar] [CrossRef]
- Barney, J.N.; Tharayil, N.; DiTommaso, A.; Bhowmik, P.C. The Biology of Invasive Alien Plants in Canada. 5. Polygonum Cuspidatum Sieb. & Zucc. [=Fallopia Japonica (Houtt.) Ronse Decr.]. Can. J. Plant Sci. 2006, 86, 887–906. [Google Scholar]
- Bailey, J. The Japanese Knotweed Invasion Viewed as a Vast Unintentional Hybridisation Experiment. Heredity 2013, 110, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Gillies, S.; Clements, D.R.; Grenz, J. Knotweed (Fallopia Spp.) Invasion of North America Utilizes Hybridization, Epigenetics, Seed Dispersal (Unexpectedly), and an Arsenal of Physiological Tactics. Invasive Plant Sci. Manag. 2016, 9, 71–80. [Google Scholar] [CrossRef]
- Sołtysiak, J.; Brej, T. Characteristics That Make the Fallopia Genus (Polygonaceae) Highly Invasive. Ecol. Quest. 2012, 16, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Michalet, S.; Rouifed, S.; Pellassa-Simon, T.; Fusade-Boyer, M.; Meiffren, G.; Nazaret, S.; Piola, F. Tolerance of Japanese Knotweed Sl to Soil Artificial Polymetallic Pollution: Early Metabolic Responses and Performance during Vegetative Multiplication. Environ. Sci. Pollut. Res. 2017, 24, 20897–20907. [Google Scholar] [CrossRef]
- Richards, C.L.; Walls, R.L.; Bailey, J.P.; Parameswaran, R.; George, T.; Pigliucci, M. Plasticity in Salt Tolerance Traits Allows for Invasion of Novel Habitat by Japanese Knotweed s. l. (Fallopia Japonica and F.×bohemica, Polygonaceae). Am. J. Bot. 2008, 95, 931–942. [Google Scholar] [CrossRef]
- Tiébré, M.-S.; Saad, L.; Mahy, G. Landscape Dynamics and Habitat Selection by the Alien Invasive Fallopia (Polygonaceae) in Belgium. Biodivers. Conserv. 2008, 17, 2357–2370. [Google Scholar] [CrossRef] [Green Version]
- Baxendale, V.J.; Tessier, J.T. Duration of Freezing Necessary to Damage the Leaves of Fallopia Japonica (H Outt.) R Onse D Ecraene. Plant Species Biol. 2015, 30, 279–284. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Johnson, C.J.; Gillingham, M.P. An Evaluation of Mapped Species Distribution Models Used for Conservation Planning. Environ. Conserv. 2005, 32, 117–128. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat Distribution Models in Ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Václavík, T.; Meentemeyer, R.K. Invasive Species Distribution Modeling (ISDM): Are Absence Data and Dispersal Constraints Needed to Predict Actual Distributions? Ecol. Model. 2009, 220, 3248–3258. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Pyšek, P.; Midgley, G.F.; Hughes, G.O.; Rouget, M. Niche-based Modelling as a Tool for Predicting the Risk of Alien Plant Invasions at a Global Scale. Glob. Change Biol. 2005, 11, 2234–2250. [Google Scholar] [CrossRef]
- Gallien, L.; Douzet, R.; Pratte, S.; Zimmermann, N.E.; Thuiller, W. Invasive Species Distribution Models-How Violating the Equilibrium Assumption Can Create New Insights. Glob. Ecol. Biogeogr. 2012, 21, 1126–1136. [Google Scholar] [CrossRef]
- Donaldson, J.E.; Richardson, D.M.; Wilson, J.R. Scale-Area Curves: A Tool for Understanding the Ecology and Distribution of Invasive Tree Species. Biol. Invasions 2014, 16, 553–563. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M.; Pergl, J.; Jarošík, V.; Sixtova, Z.; Weber, E. Geographical and Taxonomic Biases in Invasion Ecology. Trends Ecol. Evol. 2008, 23, 237–244. [Google Scholar] [CrossRef]
- Renco, M.; Čerevková, A.; Sasanelli, N. Effects of Invasive Japanese Knotweed on Diversity and Structure of Soil Nematode Communities; Institute of Zoology: Chișinău, Moldova, 2021; pp. 264–268. [Google Scholar]
- Mereďa, P.; Koláriková, Z.; Hodálová, I. Cytological and Morphological Variation of Fallopia Sect. Reynoutria Taxa (Polygonaceae) in the Krivánska Malá Fatra Mountains (Slovakia). Biologia 2019, 74, 215–236. [Google Scholar] [CrossRef]
- Lukovičová, M.; Balanac, Z.; David, S. Changes in Habitat Conditions of Invaded Forest Communities in Podunajská Nížina and the Impact of Non-Native Species on Biodiversity (SW Slovakia). Ekológia 2021, 40, 364–378. [Google Scholar] [CrossRef]
- Jovanović, S.; Hlavati-Širka, V.; Lakušić, D.; Jogan, N.; Nikolić, T.; Anastasiu, P.; Vladimirov, V.; Šinžar-Sekulić, J. Reynoutria Niche Modelling and Protected Area Prioritization for Restoration and Protection from Invasion: A Southeastern Europe Case Study. J. Nat. Conserv. 2018, 41, 1–15. [Google Scholar] [CrossRef]
- Pěknicová, J.; Petrus, D.; Berchová-Bímová, K. Application Natura 2000 Data for the Invasive Plants Spread Prediction. Sci. Agric. Bohem. 2015, 46, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Pěknicová, J.; Berchová-Bímová, K. Application of Species Distribution Models for Protected Areas Threatened by Invasive Plants. J. Nat. Conserv. 2016, 34, 1–7. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Who Cites Who in the Invasion Zoo: Insights from an Analysis of the Most Highly Cited Papers in Invasion Ecology; Preslia: Prague, Czech Republic, 2006; Volume 78, pp. 437–468. [Google Scholar]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Senay, S.D.; Worner, S.P.; Ikeda, T. Novel Three-Step Pseudo-Absence Selection Technique for Improved Species Distribution Modelling. PLoS ONE 2013, 8, e71218. [Google Scholar] [CrossRef] [Green Version]
- Brotons, L.; Thuiller, W.; Araújo, M.B.; Hirzel, A.H. Presence-absence versus Presence-only Modelling Methods for Predicting Bird Habitat Suitability. Ecography 2004, 27, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Wisz, M.S.; Guisan, A. Do Pseudo-Absence Selection Strategies Influence Species Distribution Models and Their Predictions? An Information-Theoretic Approach Based on Simulated Data. BMC Ecol. 2009, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Soberón, J.; Peterson, A.T. Interpretation of Models of Fundamental Ecological Niches and Species’ Distributional Areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tyser, R.W.; Worley, C.A. Alien Flora in Grasslands Adjacent to Road and Trail Corridors in Glacier National Park, Montana (USA). Conserv. Biol. 1992, 6, 253–262. [Google Scholar] [CrossRef]
- Lonsdale, W.; Lane, A. Tourist Vehicles as Vectors of Weed Seeds in Kakadu National Park, Northern Australia. Biol. Conserv. 1994, 69, 277–283. [Google Scholar] [CrossRef]
- Parendes, L.A.; Jones, J.A. Role of Light Availability and Dispersal in Exotic Plant Invasion along Roads and Streams in the H.J. Andrews Experimental Forest, Oregon. Conserv. Biol. 2000, 14, 64–75. [Google Scholar] [CrossRef]
- Bímová, K.; Mandák, B.; Kašparová, I. How Does Reynoutria Invasion Fit the Various Theories of Invasibility? J. Veg. Sci. 2004, 15, 495–504. [Google Scholar] [CrossRef]
- Navratil, O.; Brekenfeld, N.; Puijalon, S.; Sabastia, M.; Boyer, M.; Pella, H.; Lejot, J.; Piola, F. Distribution of Asian Knotweeds on the Rhône River Basin, France: A Multi-Scale Model of Invasibility That Combines Biophysical and Anthropogenic Factors. Sci. Total Environ. 2021, 763, 142995. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.M.; van Boheman, H.; Whelan, P.; Akbar, K.; O’Malley, V.; O’Leary, G.; Keizer, P. Towards the Sustainable Development of Modern Road in the Ecology of Transportation: Managing Mobility for the Environment; Davenport, J., Davenport, J.L., Eds.; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2006; pp. 275–331. ISBN 978-1-4020-4504-2. [Google Scholar]
- Dassonville, N.; Guillaumaud, N.; Piola, F.; Meerts, P.; Poly, F. Niche Construction by the Invasive Asian Knotweeds (Species Complex Fallopia): Impact on Activity, Abundance and Community Structure of Denitrifiers and Nitrifiers. Biol. Invasions 2011, 13, 1115–1133. [Google Scholar] [CrossRef]
- Parepa, M.; Schaffner, U.; Bossdorf, O. Help from under Ground: Soil Biota Facilitate Knotweed Invasion. Ecosphere 2013, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bardon, C.; Piola, F.; Bellvert, F.; Haichar, F.E.Z.; Comte, G.; Meiffren, G.; Pommier, T.; Puijalon, S.; Tsafack, N.; Poly, F. Evidence for Biological Denitrification Inhibition (BDI) by Plant Secondary Metabolites. New Phytol. 2014, 204, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Murrell, C.; Gerber, E.; Krebs, C.; Parepa, M.; Schaffner, U.; Bossdorf, O. Invasive Knotweed Affects Native Plants through Allelopathy. Am. J. Bot. 2011, 98, 38–43. [Google Scholar] [CrossRef]
- Parepa, M.; Bossdorf, O. Testing for Allelopathy in Invasive Plants: It All Depends on the Substrate! Biol. Invasions 2016, 18, 2975–2982. [Google Scholar] [CrossRef]
- Rouifed, S.; Puijalon, S.; Viricel, M.-R.; Piola, F. Achene Buoyancy and Germinability of the Terrestrial Invasive Fallopia× Bohemica in Aquatic Environment: A New Vector of Dispersion? Ecoscience 2011, 18, 79–84. [Google Scholar] [CrossRef]
- Martin, F.-M. Apports de l’Étude Multiscalaire Des Dynamiques Spatiales Des Renouées Asiatiques (Reynoutria Spp.) Pour l’Amélioration de La Gestion The Study of the Spatial Dynamics of Asian Knotweeds (Reynoutria Spp.) across Scales and Its Contribution for Manag. PhD Thesis, Université Grenoble Alpes (ComUE), Saint-Martin-d’Hères, France, 2019; p. 138. [Google Scholar]
- Buffa, G.; Gaetan, C.; Piccoli, S.; Del Vecchio, S.; Fantinato, E. Using Fine-Scale Field Data Modelling for Planning the Management of Invasions of Oenothera Stucchii in Coastal Dune Systems. Ecol. Indic. 2021, 125, 107564. [Google Scholar] [CrossRef]
- Manzoor, S.A.; Griffiths, G.; Lukac, M. Species Distribution Model Transferability and Model Grain Size-Finer May Not Always Be Better. Sci. Rep. 2018, 8, 7168. [Google Scholar] [CrossRef] [Green Version]
- Pyšek, P.; Prach, K. Plant Invasions and the Role of Riparian Habitats: A Comparison of Four Species Alien to Central Europe. In Ecosystem Management; Springer: Berlin/Heidelberg, Germany, 1993; pp. 254–263. [Google Scholar]
- Pyle, W. Riparian Habitat Restoration at Hart Mountain National Antelope Refuge. Restor. Manag. Notes 1995, 13, 40–44. [Google Scholar] [CrossRef]
- Chytrý, M.; Wild, J.; Pyšek, P.; Tichý, L.; Danihelka, J.; Knollová, I. Maps of the Level of Invasion of the Czech Republic by Alien Plants. Preslia 2009, 81, 187–207. [Google Scholar]
- Richardson, D.; Holmes, P.; Esler, K.; Galatowitsch, S.; Stromberg, J.; Kirkman, S.; Pyšek, P.; Hobbs, R. Riparian Vegetation: Degradation, Alien Plant Invasions, and Restoration Prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- Brock, J.; Wade, M. Regeneration of Japanese Knotweed (Fallopia Japonica) from Rhizomes and Stems: Observation from Greenhouse Trials; ANPP: Dijon, France, 1992; pp. 85–94. [Google Scholar]
- Child, L.E. Vegetative Regeneration and Distribution of Fallopia Japonica and Fallopia x Bohemica: Implications for Control and Management. Ph.D. Thesis, Loughborough University, Loughborough, UK, 1999. [Google Scholar]
- Lammeranner, W.; Schmidt, C.; Eitler, M.; Natascha, S. The Control of Invasive Knotweed Species (Fallopia sp.); Research Experiences: Vienna, Austria, 2013; p. EGU2013-11598. [Google Scholar]
- Colleran, B.; Lacy, S.N.; Retamal, M.R. Invasive Japanese Knotweed (Reynoutria Japonica Houtt.) and Related Knotweeds as Catalysts for Streambank Erosion. River Res. Appl. 2020, 36, 1962–1969. [Google Scholar] [CrossRef]
- Thomas, S.M.; Verhoeven, M.R.; Walsh, J.R.; Larkin, D.J.; Hansen, G.J. Species Distribution Models for Invasive Eurasian Watermilfoil Highlight the Importance of Data Quality and Limitations of Discrimination Accuracy Metrics. Ecol. Evol. 2021, 11, 12567–12582. [Google Scholar] [CrossRef] [PubMed]
- Bourchier, R.S.; Hezewijk, B.H.V. Distribution and Potential Spread of Japanese Knotweed (Polygonum Cuspidatum) in Canada Relative to Climatic Thresholds. Invasive Plant. Sci. Manag. 2010, 3, 32–39. [Google Scholar] [CrossRef]
- Miklos, L. Landscape Atlas of the Slovak Republic; Ministry of Environment of the Slovak Republic, Slovak Environmental Agency: Banska Bystrica, Slovakia, 2002; ISBN 978-80-88833-27-7.
- Čistoňová, Z.; Sofková, M.; David, S. Výskyt Inváznych Druhov Rodu Fallopia Na Slovensku. In Proceedings of the Študentská Vedecká Konferencia, Nitra, Slovakia, 26 April 2017. [Google Scholar]
- Neeti, N.; Václavík, T.; Niphadkar, M. Potential Distribution of Japanese Knotweed (Polygonum Cuspidatum) in Massachusetts. Methods 2007, 4, 5. [Google Scholar]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD–a Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. Sdm: A Reproducible and Extensible R Platform for Species Distribution Modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]




| Method | AUC | COR | TSS | Deviance |
|---|---|---|---|---|
| glm | 0.90 | 0.63 | 0.7 | 0.75 |
| rf | 0.94 | 0.76 | 0.82 | 0.53 |
| maxent | 0.94 | 0.73 | 0.79 | 0.92 |
| glmnet | 0.92 | 0.55 | 0.77 | 2.78 |
| brt | 0.94 | 0.74 | 0.8 | 0.66 |
| svm | 0.92 | 0.69 | 0.76 | 0.65 |
| mars | 0.93 | 0.75 | 0.8 | 0.62 |
| rbf | 0.90 | 0.62 | 0.71 | 0.76 |
| gam | 0.93 | 0.73 | 0.79 | 0.87 |
| ranger | 0.94 | 0.75 | 0.81 | 0.52 |
| Term | Estimate | Std. Error | Statistic (z Value) | p-Value |
|---|---|---|---|---|
| (Intercept) | 2.24 | 0.417 | 5.39 | 7.21 × 10−8 *** |
| Distance from transport lines | −0.00222 | 0.000339 | −6.56 | 5.29 × 10−11 *** |
| Soil type: Fluvisols | 2.17 | 0.451 | 4.82 | 1.40 × 10−6 *** |
| Soil type: Haplic Luvisols | −1.25 | 0.570 | −2.20 | 2.81 × 10−2 * |
| Soil type: Leptosols | 0.203 | 0.848 | 0.239 | 8.11 × 10−1 |
| Soil type: Mollic Fluvisols and Mollic Gleysols | −0.363 | 0.611 | −0.594 | 5.52 × 10−1 |
| Soil type: Planosols and Stagnosols | 0.681 | 0.585 | 1.17 | 2.44 × 10−1 |
| Distance from water bodies | −0.000250 | 0.0000875 | −2.85 | 4.34 × 10−3 ** |
| ID | Layer | Description | Type | VIF | Source |
|---|---|---|---|---|---|
| 1. | Transport_dist | Euclidean proximity map of roads and rails (range: 10,573 m; mean: 1159 ± 1190 m) | Continuous | 1.64 | Institute of Landscape Ecology of SAS |
| 2. | Aspect | Categorized aspect directions | Categorical (n = 8) | 1.01 | Derived from DEM |
| 3. | CLC | CORINE Land Cover 2018 (hierarchical 3-level CLC nomenclature) | Categorical (n = 31) | 1.44 | EEA (2018) |
| 4. | Landform | Type of slope landform | Categorical (n = 36) | 1.63 | Institute of Landscape Ecology of SAS |
| 5. | Soil_texture | Soil texture | Categorical (n = 12) | 1.08 | Institute of Landscape Ecology of SAS |
| 6. | Soil_type | Soil type | Categorical (n = 22) | 1.42 | Institute of Landscape Ecology of SAS |
| 7. | Rivers_dist | Euclidean proximity to rivers (range: 6937 m; mean: 348 ± 390 m) | Continuous | 1.25 | Institute of Landscape Ecology of SAS |
| 8. | DEM | Digital elevation model (range: 2521 asl; mean: 454 ± 313 m asl) | Continuous | 4.08 | EEA (2018) |
| 9. | Slope | Surface slope (range: 76°; mean: 9 ± 8° m) | Continuous | 1.62 | Derived from DEM |
| 10. | Water_bodies_dist | Euclidean proximity map of waterbodies (range: 17,430 m; mean: 3228 ± 2219 m) | Continuous | 1.18 | Institute of Landscape Ecology of SAS |
| 11. | Min_temp_01 | Minimum temperature in January (range: 7 °C; mean: −8±1 °C) | Continuous | 2.42 | Fick and Hijmans, 2017 (WorldClim) |
| 12. | Precipitation | Precipitation (range: 1184 mm; mean: 734 ± 169 mm) | Continuous | 2.57 | Fick and Hijmans, 2017 (WorldClim) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gašparovičová, P.; Ševčík, M.; David, S. The Prediction of Distribution of the Invasive Fallopia Taxa in Slovakia. Plants 2022, 11, 1484. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11111484
Gašparovičová P, Ševčík M, David S. The Prediction of Distribution of the Invasive Fallopia Taxa in Slovakia. Plants. 2022; 11(11):1484. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11111484
Chicago/Turabian StyleGašparovičová, Petra, Michal Ševčík, and Stanislav David. 2022. "The Prediction of Distribution of the Invasive Fallopia Taxa in Slovakia" Plants 11, no. 11: 1484. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11111484