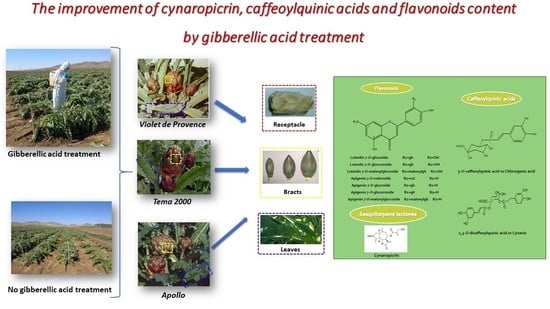
Improvement in the Cynaropicrin, Caffeoylquinic Acid and Flavonoid Content of Globe Artichokes with Gibberellic Acid Treatment
Abstract
:1. Introduction
2. Results
2.1. Polyphenol Profile Characterization
2.2. Earliness, Head Characteristics and Yield Response
3. Discussion
4. Materials and Methods
4.1. Experimental Field Design, Meteorological Conditions, Soil Characteristics, Crop Management and Plant Material
4.2. Earliness, Head Morphology, and Yield Responses
4.3. Polyphenol Profile Characterization
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lombardo, S.; Pandino, G.; Mauro, R.; Mauromicale, G. Variation of phenolic content in globe artichoke in relation to biological, technical and environmental factors. Ital. J. Agron. 2009, 4, 181–189. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT—Food and Agriculture Organization of the United Nations. Statistics Division. 2020. Available online: http://www.faostat.org (accessed on 22 May 2022).
- Lombardo, S.; Pandino, G.; Mauromicale, G. The influence of pre-harvest factors on the quality of globe artichoke. Sci. Hortic. 2018, 233, 479–490. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauro, R.P.; Mauromicale, G. Variation in polyphenol profile and head morphology among clones of globe artichoke selected from a landrace. Sci. Hortic. 2012, 138, 259–265. [Google Scholar] [CrossRef]
- Pandino, G.; Mauromicale, G. Globe artichoke and cardoon forms between traditional and modern uses. Acta Hortic. 2020, 1284, 1–18. [Google Scholar] [CrossRef]
- Pesce, G.R.; Mauromicale, G. Cynara cardunculus L.: Historical and economic importance, botanical descriptions, genetic resources and traditional uses. In The Globe Artichoke Genome. Compendium of Plant Genomes, 1st ed.; Portis, E., Acquadro, A., Lanteri, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Minerals profile of two globe artichoke cultivars as affected by NPK fertilizer regimes. Food Res. Int. 2017, 100, 95–99. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Ruta, C.; Mauromicale, G. In vitro micropropagation and mycorrhizal treatment influences the polyphenols content profile of globe artichoke under field conditions. Food Res. Int. 2017, 99, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Pereira, C.; Ntatsi, G.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and chemical composition of Greek artichoke genotypes. Food Chem. 2017, 267, 296–302. [Google Scholar] [CrossRef]
- Brat, P.; Georgé, S.; Bellamy, A.; Du Chaffaut, L.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef] [Green Version]
- Scavo, A.; Pandino, G.; Restuccia, A.; Lombardo, S.; Pesce, G.R.; Mauromicale, G. Allelopathic potential of leaf aqueous extracts from Cynara cardunculus L. on the seedling growth of two cosmopolitan weed species. Ital. J. Agron. 2019, 14, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Salata, A.; Lombardo, S.; Pandino, G.; Mauromicale, G.; Buczkowska, A.; Nurzynska-Wierdak, R. Biomass yield and polyphenol compounds profile in globe artichoke as affected by irrigation frequency and drying temperature. Ind. Crops Prod. 2022, 176, 114375. [Google Scholar] [CrossRef]
- Maroto, J.V. Effects of gibberellic acid (GA3) applications on globe artichoke production. Acta Hortic. 2007, 730, 137–142. [Google Scholar] [CrossRef]
- Mauromicale, G.; Ierna, A. Effects of gibberellic acid and sowing dates on harvest time and yields of seed-grown globe artichoke (Cynara scolymus L.). Agronomie 1995, 15, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Mauromicale, G.; Ierna, A. Characteristics of heads of seed-grown globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori] as affected by harvest period, sowing date and gibberellic acid. Agronomie 2000, 20, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Baixauli, C.; Giner, A.; Miquel, A.; López, S.; San Bautista, A.; Maroto, J.V. Interaction between cultivar and gibberellic acid concentration in seed propagated artichoke. Acta Hortic. 2007, 630, 165–170. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.A.; Schnitzler, W.H.; Nitz, G.; El-Oksh, I.I. Effects of GA3 on growth characteristics, earliness, yield, carbohydrates, and protein in globe artichoke (Cynara cardunculus var. scolymus (L.) Fiori). J. Appl. Bot. Food Qual. 2003, 77, 1–9. [Google Scholar]
- Sharaf-Eldin, M.A.; Schnitzler, W.H.; Nitz, G.; Razin, A.M.; El Oksh, I.I. The effect of gibberellic acid (GA3) on some phenolic substances in globe artichoke (Cynara cardunculus var. scolymus (L.) Fiori). Sci. Hortic. 2007, 111, 326–329. [Google Scholar] [CrossRef]
- Foury, C. Le deuxiéme congrés internacional de l’Artichaut. P.H.M. Rev. Hortic. 1974, 145, 31–36. [Google Scholar]
- Basnizki, Y. Cynara scolymus. In The Handbook of Flowering; Halevy, A.H., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 391–399. [Google Scholar]
- Bianco, V.V. Carciofo (Cynara scolymus L.). In Orticoltura; Bianco, V.V., Pimpini, F., Eds.; Patron Editore: Bologna, Italy, 1990; pp. 209–251. [Google Scholar]
- Paradiso, R.; Cuocolo, B.; De Pascale, S. Gibberellic acid and nitrogen rate affect yield and quality of artichoke. Acta Hortic. 2007, 730, 211–216. [Google Scholar] [CrossRef]
- El-Abagy, H.M.H.; Rashad El-Sh, M.; Abdel-Mawgoud, A.M.R.; El-Greadly, N.H.M. Physiological and biochemical effects of some bioregulators on growth: Productivity and quality of artichoke (Cynara scolymus L.) plant. Res. J. Agric. Biol. Sci. 2010, 6, 683–690. [Google Scholar]
- Giménez, M.J.; Giménez-Berenguer, M.; García-Pastor, M.E.; Parra, J.; Zapata, P.J.; Castillo, S. The Influence of flower head order and gibberellic acid treatment on the hydroxycinnamic acid and luteolin derivatives content in globe artichoke cultivars. Foods 2021, 10, 1813. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Mauromicale, G. Choice of time of harvest influences the polyphenol profile of globe artichoke. J. Funct. Foods 2013, 5, 1822–1828. [Google Scholar] [CrossRef]
- Ferro, A.M.; Ramos, P.; Guerreiro, O.; Jerónimo, E.; Pires, I.; Capel, C.; Capel, J.; Lozano, R.; Duarte, M.F.; Oliveira, M.M.; et al. Impact of novel SNPs identified in Cynara cardunculus genes on functionality of proteins regulating phenylpropanoid pathway and their association with biological activities. BMC Genom. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baixauli, C.; Giner, A.; Aguilar, J.M.; Najera, I.; Miguel, A.; Lopez Galarza, A.; Pascual, B.; San Bautista, A.; Maroto, J.V. Agronomic behaviour of seed propagated artichoke cultivars in the Spanish Mediterranean area. Acta Hortic. 2012, 942, 361–367. [Google Scholar] [CrossRef]
- El-Hameid, A.; Kasim, A.T.; El-Zohery, S.S.M. Effect of vernalization and gibberellic acid on earliness, total yield and quality of globe artichoke. Ann. Agric. Sci. Moshtohor. 2008, 46, 511–523. [Google Scholar]
- El-Zohiri, S.S. Performance comparison of three alternatives for GA3 on growth, earliness and total yield of globe artichoke. Middle East J. Appl. Sci. 2015, 5, 636–644. [Google Scholar]
- Mauromicale, G.; Ierna, A.; Cavallaro, V. Effects of vernalization and gibberellic acid on bolting, harvest time and yield of seed-grown globe artichoke. Acta Hortic. 2005, 681, 243–249. [Google Scholar] [CrossRef]
- Salata, A.; Gruszecki, R.; Dyduch, J. Morphological and qualitative characterization of globe artichoke (Cynara scolymus L.) cultivars ‘Symphony’ and ‘Madrigal’ on depending of the heads growth. Acta Sci. Pol.-Hortorum Cultus 2012, 11, 67–80. [Google Scholar]
- Saleh, S.A.; AEL-R, G.H.; El-Bassyouni, M.S.; Abdalla, A.M. Effect of gibberellic acid and micronutrients foliar applications on different seed-propagated artichokes under Egyptian condition. Egypt. J. Basic Appl. Sci. 2017, 32, 615–634. [Google Scholar]
- Basnizki, J.; Goldschmidt, E.E. Further examination of gibberellin GA3 effects on flowering of globe artichokes (Cynara scolymus L.) under controlled environment and field conditions. Isr. J. Plant Sci. 1994, 42, 159–166. [Google Scholar] [CrossRef]
- Dumičić, G.; Goreta Ban, S.; Bućan, L.; Borošić, J.; Poljak, M. Effect of gibberellic acid application on growth and yield of artichokes under summer conditions. J. Food Agric. Environ. 2009, 7, 620–626. [Google Scholar] [CrossRef]
- USDA. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; Handbook 436; USDA-Natural Resources Conservation Service: Washington, DC, USA, 1999; p. 869.
- Basnizki, Y.; Goldschmidt, E.; Luria, Y.; Itach, H.; Berg, Z.; Galili, D. Effect of acidified GA3-spray on yield of globe artichoke (Cynara scolymus L). Hasadeh 1986, 66, 1814–1817, (In Hebrew, English Summary). [Google Scholar]
- Regione Siciliana. Disciplinare Regionale Produzione Integrata; Regione Siciliana: Palermo, Italy, 2016; pp. 201–206. Available online: https://pti.regione.sicilia.it (accessed on 20 May 2022).
- Pandino, G.; Meneghini, M.; Tavazza, R.; Lombardo, S.; Mauromicale, G. Phytochemicals accumulation and antioxidant activity in callus and suspension cultures of Cynara scolymus L. Plant Cell Tissue Organ Cult. 2017, 128, 223–230. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, C.; Parafati, L.; Cirvilleri, G.; Mauromicale, G. Antimicrobial activity of cultivated cardoon (Cynara cardunculus L. var. altilis DC.) leaf extracts against bacterial species of agricultural and food interest. Ind. Crops Prod. 2019, 129, 206–211. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Characterization of phenolic acids and flavonoids in leaves, stems, bracts and edible parts of globe artichokes. Acta Hortic. 2012, 942, 413–417. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.; Carle, R.; Schieber, A. Identification and quantification of caffeoylquinic acids and flavonoide from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 2004, 52, 4090–4096. [Google Scholar] [CrossRef]

| Parameter | Source of Variation | ||||||
|---|---|---|---|---|---|---|---|
| Treatment (T) | Genotype (G) | Harvest Time (H) | T × G | T × H | G × H | T × G × H | |
| FSs Tot CQA | 24.9 *** | 8.4 * | 31.3 *** | 13.2 *** | 8.9 * | 12.4 *** | 0.9 ns |
| FSs Tot Api | 18.6 *** | 29.7 *** | 18.4 *** | 0.5 ns | 20.8 *** | 10.5 ** | 0.6 ns |
| FSs Tot Lut | 17.2 *** | 17.2 *** | 22.2 *** | 14.8 *** | 1.7 ns | 15.9 *** | 1.0 ns |
| FSs Tot Phe | 29.0 *** | 25.9 *** | 15.2 *** | 4.9 * | 14.4 *** | 8.4 ** | 2.2 ns |
| REs Tot CQA | 11.5 *** | 43.6 *** | 12.5 *** | 13.7 *** | 16.7 *** | 0.5 ns | 1.5 ns |
| REs Tot Api | 1.9 ns | 43.3 *** | 25.1 *** | 22.6 *** | 3.0 ns | 0.4 ns | 3.5 ns |
| REs Tot Lut | 16.1 *** | 19.0 *** | 32.0 *** | 10.1 ** | 5.7 ns | 15.2 *** | 0.9 ns |
| REs Tot Phe | 18.9 *** | 33.5 *** | 23.2 *** | 0.8 ns | 17.7 *** | 2.8 ns | 3.1 ns |
| LEs Tot CQA | 0.5 ns | 10.1 ** | 8.0 * | 1.7 ns | 18.6 ** | 59.1 *** | 2.0 ns |
| LEs Tot Api | 38.7 *** | 37.1 *** | 7.5 * | 2.2 ns | 5.6 ns | 5.2 ns | 3.7 ns |
| LEs Tot Lut | 26.7 *** | 24.4 *** | 21.0 *** | 0.2 ns | 15.3 ** | 12.2 ** | 0.2 ns |
| LEs Tot Phe | 16.5 *** | 20.6 *** | 11.3 ** | 12.0 ** | 26.7 *** | 12.5 ** | 0.4 ns |
| LEs Cyn | 12.7 ** | 9.9 ** | 31.4 *** | 10.2 ** | 11.7 ** | 21.2 *** | 2.9 ns |
| Df | 1 | 2 | 1 | 2 | 1 | 2 | 2 |
| Parameter | H1 | H2 | ||
|---|---|---|---|---|
| GA3 | Control | GA3 | Control | |
| FSs Tot CQA | 14.65 ± 0.21 a | 7.23 ± 0.26 b | 5.89 ± 0.26 c | 4.02 ± 0.15 d |
| FSs Tot Api | 0.56 ± 0.06 b | 0.31 ± 0.06 c | 0.95 ± 0.09 a | 0.59 ± 0.04 b |
| FSs Tot Lut | 0.61 ± 0.04 | 0.24 ± 0.02 | 3.37 ± 0.16 | 5.25 ± 0.15 |
| FSs Tot Phe | 15.82 ± 0.22 a | 7.79 ± 0.10 c | 10.21 ± 0.28 b | 9.86 ± 0.21 b |
| REs Tot CQA | 2.10 ± 0.13 b | 1.61 ± 0.16 c | 1.67 ± 0.19 c | 2.58 ± 0.12 a |
| REs Tot Api | 1.32 ± 0.05 | 1.15 ± 0.09 | 1.11 ± 0.10 | 1.18 ± 0.09 |
| REs Tot Lut | 0.23 ± 0.05 | 0.19 ± 0.07 | 0.38 ± 0.06 | 0.49 ± 0.03 |
| REs Tot Phe | 3.65 ± 0.14 b | 2.96 ± 0.19 c | 3.17 ± 0.11 c | 4.24 ± 0.13 a |
| LEs Tot CQA | 1.42 ± 0.09 a | 0.92 ± 0.10 c | 0.86 ± 0.06 c | 1.22 ± 0.16 b |
| LEs Tot Api | 0.44 ± 0.02 | 0.90 ± 0.09 | 0.53 ± 0.06 | 0.89 ± 0.07 |
| LEs Tot Lut | 5.86 ± 0.20 a | 2.29 ± 0.14 d | 3.19 ± 0.16 c | 4.36 ± 0.15 b |
| LEs Tot Phe | 7.71 ± 0.16 a | 4.11 ± 0.10 c | 4.58 ± 0.15 c | 6.47 ± 0.16 b |
| LEs Cyn | 2.10 ± 0.11 b | 1.70 ± 0.07 c | 3.48 ± 0.15 a | 2.61 ± 0.09 b |
| Parameter | GA3 | Control | ||||
|---|---|---|---|---|---|---|
| Apollo | Tema 2000 | Violet de Provence | Apollo | Tema 2000 | Violet de Provence | |
| FSs Tot CQA | 8.56 ± 0.59 c | 11.03 ± 1.01 a | 11.22 ± 1.02 a | 3.17 ± 0.30d | 3.97 ± 0.19 d | 9.73 ± 1.00 b |
| FSs Tot Api | 0.27 ± 0.02 | 1.41 ± 0.10 | 0.58 ± 0.05 | 0.24 ± 0.02 | 0.58 ± 0.03 | 0.53 ± 0.03 |
| FSs Tot Lut | 0.71 ± 0.05 d | 3.23 ± 0.22 b | 2.03 ± 0.15 c | 0.28 ± 0.05 e | 5.13 ± 0.48 a | 2.83 ± 0.24 b |
| FSs Tot Phe | 9.54 ± 0.68 c | 15.67 ± 1.02 a | 13.84 ± 1.19 b | 3.69 ± 0.21 d | 9.69 ± 0.28 c | 13.09 ± 0.46 b |
| REs Tot CQA | 1.20 ± 0.12 de | 1.48 ± 0.09 cd | 2.97 ± 0.23 b | 0.99 ± 0.10 e | 1.89 ± 0.11 c | 3.40 ± 0.26 a |
| REs Tot Api | 1.05 ± 0.05 | 1.05 ± 0.06 | 1.55 ± 0.08 | 0.65 ± 0.03 | 1.33 ± 0.10 | 1.52 ± 0.12 |
| REs Tot Lut | 0.49 ± 0.02 | 0.07 ± 0.01 | 0.36 ± 0.03 | 0.33 ± 0.03 | 0.32 ± 0.04 | 0.37 ± 0.03 |
| REs Tot Phe | 2.74 ± 0.21 d | 2.60 ± 0.15 d | 4.88 ± 0.41 b | 1.98 ± 0.12 e | 3.53 ± 0.21 c | 5.29 ± 0.28 a |
| LEs Tot CQA | 1.44 ± 0.10 | 1.10 ± 0.15 | 0.86 ± 0.05 | 1.39 ± 0.51 | 0.80 ± 0.06 | 1.02 ± 0.08 |
| LEs Tot Api | 0.44 ± 0.03 | 0.27 ± 0.02 | 0.74 ± 0.06 | 0.36 ± 0.02 | 1.62 ± 0.11 | 0.70 ± 0.04 |
| LEs Tot Lut | 5.67 ± 0.40 a | 3.54 ± 0.30 c | 4.36 ± 0.25 b | 4.22 ± 0.32 b | 2.53 ± 0.09 d | 3.22 ± 0.04 c |
| LEs Tot Phe | 7.56 ± 0.21 a | 4.91 ± 0.28 c | 5.96 ± 0.39 b | 5.97 ± 0.28 b | 4.95 ± 0.18 c | 4.94 ± 0.31 c |
| LEs Cyn | 2.47 ± 0.18 c | 3.29 ± 0.15 a | 2.60 ± 0.23 bc | 1.80 ± 0.10 d | 2.76 ± 0.05 b | 1.91 ± 0.09 d |
| Parameter | H1 | H2 | ||||
|---|---|---|---|---|---|---|
| Apollo | Tema 2000 | Violet de Provence | Apollo | Tema 2000 | Violet de Provence | |
| FSs Tot CQA | 5.59 ± 0.30 c | 12.31 ± 1.06 b | 14.93 ± 1.05 a | 6.14 ± 0.38 c | 2.70 ± 0.15 d | 6.03 ± 0.19 c |
| FSs Tot Api | 0.30 ± 0.02 c | 0.85 ± 0.03 b | 0.16 ± 0.01 d | 0.22 ± 0.02 cd | 1.14 ± 0.05 a | 0.95 ± 0.05 ab |
| FSs Tot Lut | 0.35 ± 0.04 c | 0.50 ± 0.01 c | 0.44 ± 0.04 c | 0.64 ± 0.06 c | 7.86 ± 0.29 a | 4.43 ± 0.14 b |
| FSs Tot Phe | 6.24 ± 0.15 d | 13.66 ± 1.01 b | 15.52 ± 0.39 a | 6.99 ± 0.26 d | 11.70 ± 1.05 c | 11.41 ± 0.59 c |
| REs Tot CQA | 1.04 ± 0.02 e | 1.54 ± 0.04 d | 2.98 ± 0.09 b | 1.15 ± 0.09 e | 1.83 ± 0.09 c | 3.39 ± 0.22 a |
| REs Tot Api | 0.47 ± 0.05 | 1.34 ± 0.08 | 1.90 ± 0.04 | 1.23 ± 0.13 | 1.04 ± 0.08 | 1.17 ± 0.09 |
| REs Tot Lut | 0.28 ± 0.01 b | 0.12 ± 0.01 c | 0.24 ± 0.03 b | 0.54 ± 0.06 a | 0.27 ± 0.01 b | 0.49 ± 0.01 a |
| REs Tot Phe | 1.79 ± 0.09 | 2.99 ± 0.14 | 5.12 ± 0.41 | 2.92 ± 0.13 | 3.14 ± 0.17 | 5.05 ± 0.23 |
| LEs Tot CQA | 0.73 ± 0.05 c | 1.30 ± 0.05 b | 1.47 ± 0.09 b | 2.10 ± 0.09 a | 0.60 ± 0.03 cd | 0.42 ± 0.01 d |
| LEs Tot Api | 0.38 ± 0.01 | 1.12 ± 0.09 | 0.51 ± 0.05 | 0.43 ± 0.03 | 0.77 ± 0.03 | 0.92 ± 0.05 |
| LEs Tot Lut | 4.66 ± 0.35 bc | 3.47 ± 0.05 d | 4.08 ± 0.25 c | 5.23 ± 0.22 a | 2.60 ± 0.21 e | 3.50 ± 0.32 d |
| LEs Tot Phe | 5.77 ± 0.30 c | 5.89 ± 0.21 c | 6.07 ± 0.19 bc | 7.76 ± 0.52 a | 3.97 ± 0.35 e | 4.84 ± 0.20 d |
| LEs Cyn | 1.99 ± 0.12 c | 1.46 ± 0.05 d | 2.24 ± 0.09 b | 2.27 ± 0.20 b | 4.59 ± 0.32 a | 2.26 ± 0.12 b |
| Compound | Treatment | Genotype | Harvest Time | ||||
|---|---|---|---|---|---|---|---|
| GA3 | Control | Apollo | Tema 2000 | Violet de Provence | H1 | H2 | |
| FSs 5-CQA | 4.76 ± 0.32 a | 3.12 ± 0.11 b | 2.03 ± 0.12 c | 3.97 ± 0.22 b | 5.81 ± 0.38 a | 5.20 ± 0.21 a | 2.68 ± 0.20 b |
| FSs 3,5-diCQA | 2.24 ± 0.15 a | 1.01 ± 0.08 b | 1.30 ± 0.11 b | 1.74 ± 0.14 a | 1.84 ± 0.12 a | 2.36 ± 0.14 a | 0.90 ± 0.08 b |
| FSs 1,5-diCQA | 3.18 ± 0.25 a | 1.44 ± 0.08 b | 2.39 ± 0.21 b | 1.83 ± 0.09 c | 2.70 ± 0.20 a | 3.32 ± 0.09 a | 1.30 ± 0.05 b |
| FSs 4,5-diCQA | 0.11 a | 0.07 b | 0.14 | nd | 0.13 | 0.07 | 0.11 |
| FSs API-7-rut | nd | 0.01 | 0.01 | tr | tr | 0.01 | tr |
| FSs API-7-glc | 0.06 a | 0.02 b | 0.11 | nd | tr | 0.07 | nd |
| FSs API-7-mal | 0.69 ± 0.05 a | 0.41 ± 0.03 b | 0.10 c | 1.00 ± 0.08 a | 0.56 ± 0.03 b | 0.34 b | 0.76 ± 0.03 a |
| FSs API | 0.07 a | 0.02 b | 0.13 | tr | tr | 0.02 b | 0.06 a |
| FSs LUT-7-glc | 1.06 ± 0.06 b | 1.66 ± 0.11 a | 0.16 c | 2.52 ± 0.23 a | 1.40 ± 0.11 b | 0.23 b | 2.49 ± 0.16 a |
| FSs LUT-7-glr | 0.75 ± 0.02 b | 1.04 ± 0.09 a | 0.03 c | 1.66 ± 0.15 a | 1.00 ± 0.05 b | 0.12 b | 1.67 ± 0.12 a |
| FSs LUT-7-mal | 0.08 a | 0.04 b | 0.14 | nd | 0.04 | 0.03 b | 0.09 a |
| FSs LUT | 0.05 | tr | 0.08 | tr | nd | 0.05 | nd |
| REs 5-CQA | 0.62 ± 0.05 | 0.61 ± 0.05 | 0.33 ± 0.01 b | 0.28 b | 1.24 ± 0.11 a | 0.56 ± 0.04 b | 0.67 a |
| REs 3,5-diCQA | 0.42 ± 0.01 b | 0.64 ± 0.03 a | 0.35 ± 0.02 b | 0.60 a | 0.65 ± 0.03 a | 0.47 b | 0.60 ± 0.04 a |
| REs 1,5-diCQA | 0.63 ± 0.01 b | 0.95 ± 0.03 a | 0.39 ± 0.01 c | 0.68 ± 0.04 b | 1.30 ± 0.09 a | 0.72 ± 0.03 b | 0.86 ± 0.03 a |
| REs API-7-rut | 0.32 ± 0.03 b | 0.63 ± 0.05 a | 0.32 c | 0.48 b | 0.63 ± 0.02 a | 0.60 a | 0.35 b |
| REs API-7-glc | 0.43 ± 0.02 a | 0.25 b | 0.23 b | 0.14 b | 0.65 ± 0.01 a | 0.38 a | 0.29 ± 0.02 b |
| REs API-7-glr | tr | 0.12 | tr | 0.08 | 0.10 | 0.08 a | 0.03 b |
| REs API | 0.27 ± 0.01 | 0.20 | 0.31 a | 0.34 ± 0.02 a | 0.06 b | 0.18 b | 0.29 a |
| REs LUT-7-glc | 0.25 ± 0.02 | 0.23 ± 0.02 | 0.28 ± 0.01 a | 0.12 b | 0.33 ± 0.01 a | 0.16 b | 0.32 ± 0.02 a |
| REs LUT-7-mal | 0.07 | 0.07 | 0.14 a | 0.04 b | 0.03 b | 0.05 b | 0.09 a |
| LEs 5-CQA | 0.68 ± 0.01 b | 0.85 ± 0.02 a | 0.90 ± 0.02 a | 0.70 ± 0.05 b | 0.70 b | 0.89 ± 0.02 a | 0.64 ± 0.01 b |
| LEs 3,5-diCQA | 0.12 | 0.11 | 0.13 | 0.12 | 0.10 | 0.11 | 0.13 |
| LEs 1,5-diCQA | 0.16 ± 0.01 b | 0.28 ± 0.01 a | 0.39 ± 0.01 a | 0.13 b | 0.14 b | 0.17 b | 0.27 a |
| LEs API-7-rut | 0.35 ± 0.02 | 0.31 | 0.21 b | 0.29 b | 0.48 a | 0.33 ± 0.01 | 0.33 ± 0.02 |
| LEs API-7-glc | 0.27 ± 0.01 | 0.25 | 0.19 b | 0.44 a | 0.15 b | 0.22 ± 0.02 b | 0.30 a |
| LEs API-7-glr | 0.16 ± 0.01 a | 0.05 b | tr | 0.22 ± 0.01 a | 0.09 b | 0.13 a | 0.08 b |
| LEs LUT-7-glc | 2.25 ± 0.15 | 2.05 ± 0.13 | 2.63 ± 0.09 a | 2.20 ± 0.11 b | 1.62 ± 0.13 c | 2.20 ± 0.17 | 2.10 ± 0.16 |
| LEs LUT-7-glr | 0.67 ± 0.02 a | 0.52 ± 0.03 b | 0.53 ± 0.03 b | 0.33 ± 0.04 c | 0.92 ± 0.08 a | 0.56 ± 0.04 | 0.63 ± 0.01 |
| LEs LUT-7-mal | 1.01 ± 0.05 | 0.90 ± 0.02 | 1.10 ± 0.08 a | 0.52 ± 0.01 b | 1.25 ± 0.09 a | 1.31 ± 0.13 a | 0.61 ± 0.05 b |
| LEs LUT | tr | 0.46 ± 0.01 | 0.69 ± 0.03 | tr | tr | tr | 0.46 ± 0.03 |
| LEs Cyn | 2.84 ± 0.09 a | 2.11 ± 0.10 b | 2.13 ± 0.15 b | 3.02 ± 0.23 a | 2.26 ± 0.10 b | 1.90 ± 0.06 b | 3.04 ± 0.16 a |
| Parameter | Source of Variation | ||
|---|---|---|---|
| Treatment (T) | Genotype (G) | T × G | |
| Earliness | 14.0 *** | 79.2 *** | 6.8 *** |
| Number of heads plant−1 | 66.0 *** | 10.9 *** | 23.1 *** |
| Fresh weight yield | 12.2 * | 72.9 *** | 14.9 * |
| Mean head fresh weight | 6.2 * | 64.4 *** | 29.4 ** |
| Length/width head ratio | 11.1 * | 61.2 *** | 27.8 ** |
| Edible fraction percentage | 17.6 ** | 42.5 ** | 39.9 *** |
| Degrees of freedom | 1 | 2 | 2 |
| Genotype | Earliness (d) * | Number of Heads Plant−1 | Fresh Weight Yield (Kg Plant−1) | |||
|---|---|---|---|---|---|---|
| Control | GA3 | Control | GA3 | Control | GA3 | |
| Apollo | 234 ± 5 a | 194 ± 4 b | 12.4 ± 1.0 b | 13.3 ± 0.6 a | 3.3 ± 0.1 a | 3.5 ± 0.3 a |
| Tema 2000 | 157 ± 6 c | 130 ± 5 f | 8.1 ± 0.5 d | 13.9 ± 0.5 a | 1.1 ± 0.1 c | 1.6 ± 0.1 b |
| Violet de Provence | 144 ± 8 de | 136 ± 4 ef | 11.6 ± 0.7 c | 12.9 ± 0.4 b | 1.7 ± 0.2 b | 1.8 ± 0.2 b |
| Mean Head Fresh Weight (g) | Length/Width Head Ratio | Edible Fraction Percentage (%) | ||||
| Control | GA3 | Control | GA3 | Control | GA3 | |
| Apollo | 269 ± 5 a | 261 ± 5 a | 0.99 ± 0.02 e | 1.14 ± 0.04 d | 20.7 ± 1.1 c | 25.0 ± 1.3 a |
| Tema 2000 | 141 ± 4 cd | 119 ± 6 e | 1.40 ± 0.04 b | 1.47 ± 0.06 a | 19.7 ± 0.8 d | 20.0 ± 1.1 cd |
| Violet de Provence | 155 ± 6 bc | 132 ± 3 d | 1.32 ± 0.05 c | 1.40 ± 0.07 b | 22.0 ± 0.9 b | 21.7 ± 1.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, S.; Scavo, A.; Pandino, G.; Cantone, M.; Mauromicale, G. Improvement in the Cynaropicrin, Caffeoylquinic Acid and Flavonoid Content of Globe Artichokes with Gibberellic Acid Treatment. Plants 2022, 11, 1845. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11141845
Lombardo S, Scavo A, Pandino G, Cantone M, Mauromicale G. Improvement in the Cynaropicrin, Caffeoylquinic Acid and Flavonoid Content of Globe Artichokes with Gibberellic Acid Treatment. Plants. 2022; 11(14):1845. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11141845
Chicago/Turabian StyleLombardo, Sara, Aurelio Scavo, Gaetano Pandino, Marco Cantone, and Giovanni Mauromicale. 2022. "Improvement in the Cynaropicrin, Caffeoylquinic Acid and Flavonoid Content of Globe Artichokes with Gibberellic Acid Treatment" Plants 11, no. 14: 1845. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11141845