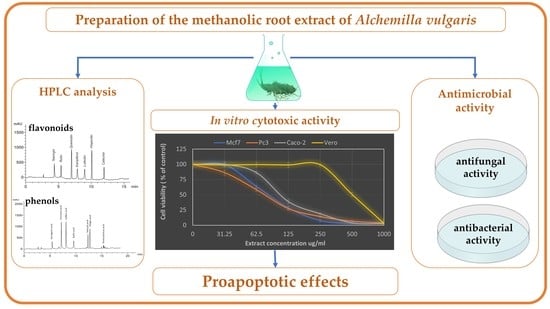
Phytochemical Characterization, Antimicrobial Activity and In Vitro Antiproliferative Potential of Alchemilla vulgaris Auct Root Extract against Prostate (PC-3), Breast (MCF-7) and Colorectal Adenocarcinoma (Caco-2) Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract Preparation
2.2. HPLC Analysis of A. vulgaris Root Extract
2.3. Antifungal Activity of A. vulgaris Root Extract
2.4. Antibacterial Activity of A. vulgaris Root Extract
2.5. Cytotoxicity Test
2.5.1. Cell Cultures
2.5.2. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Assay
2.6. Cell Cycle Arrest Assessment
2.7. Flow Cytometry Assessment of Apoptotic vs. Necrotic Cells
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Chemical Composition of A. vulgaris Root Extract
3.2. Antimicrobial Activity of A. vulgaris Root Extract
3.3. Antiproliferative Activity of A. vulgaris Root Extract
3.4. Effect of A. vulgaris Root Extract on Cell Cycle Arrest in PC-3 Cells
3.5. Effect of A. vulgaris Root Extract on Apoptosis and Necrosis of Cells
3.6. Effect of A. vulgaris Root Extract on the Expression of Apoptosis-Related Genes in PC-3 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tadić, V.M.; Krgović, N.; Ana, Ž. Lady’s mantle (Alchemilla vulgaris L., Rosaceae): A review of traditional uses, phytochemical profile, and biological properties. Nat. Med. Mater. 2020, 40, 66–74. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suarez-Álvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Boroja, T.; Mihailović, V.; Katanić, J.; Pan, S.P.; Nikles, S.; Imbimbo, P.; Monti, D.M.; Stanković, N.; Stanković, M.S.; Bauer, R. The biological activities of roots and aerial parts of Alchemilla vulgaris L. South Afr. J. Bot. 2018, 116, 175–184. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Akram, M.; Balasubramanian, I.; Tam, K.K.Y.; Koh, K.X.; Yee, M.Q.; Soong, R. Pharmacologic synergy between dual phosphoinositide-3-kinase and mammalian target of rapamycin inhibition and 5-fluorouracil in PIK3CA mutant gastric cancer cells. Cancer Biol. Ther. 2012, 13, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hafeez, E.Y.; Orabi, M.A.A.; Ibrahim, O.H.M.; Ilinskaya, O.; Karamova, N.S. In vitro cytotoxic activity of certain succulent plants against human colon, breast and liver cancer cell lines. South Afr. J. Bot. 2020, 131, 295–301. [Google Scholar] [CrossRef]
- Kovač, M.J.; Jokić, S.; Jerković, I.; Molnar, M. Optimization of deep eutectic solvent extraction of phenolic acids and tannins from Alchemilla vulgaris L. Plants 2022, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Duckstein, S.M.; Lotter, E.M.; Meyer, U.; Lindequist, U.; Stintzing, F.C. Phenolic constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at different dates of harvest. Z. für Nat. C 2012, 67, 529–540. [Google Scholar] [CrossRef]
- Jurić, T.; Katanić Stanković, J.S.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Mihailović, V. Protective effects of Alchemilla vulgaris L. extracts against cisplatin-induced toxicological alterations in rats. South Afr. J. Bot. 2020, 128, 141–151. [Google Scholar] [CrossRef]
- Jelača, S.; Drača, D.; Dajić Stevanović, Z.; Mijatović, S.; Jovanović, I.; Jovanović, M.; Jurišević, M.; Arsenijević, N.; Maksimović-Ivanić, D. Antitumor potential of Alchemilla vulgaris L. in ortotopic mouse breast cancer model. In Proceedings of the 6th European Congress of Immunology, Virtual Meeting, 1–4 September 2021; p. 351. [Google Scholar]
- Jelača, S.; Drača, D.; Dajić Stevanović, Z.; Jovanović, I.; Pavlović, S.; Gajović, N.; Mijatović, S.; Arsenijević, N.; Maksimović-Ivanić, D. Multiple effects of Alchemilla vulgaris L. extract on melanoma cells and tumor microenvironment. In Proceedings of the 6th European Congress of Immunology, Virtual Meeting, 1–4 September 2021; p. 352. [Google Scholar]
- Brulez, W.; Zeller, W. Seasonal changes of epiphytic Erwinia amylovora on ornamentals in relation to weather conditions and course of infections. Acta Hortic. 1981, 117, 37–43. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alhawassi, T.; Qamar, W.; El-Gamal, A. Cytotoxic Evaluation and Anti-Angiogenic Effects of Two Furano-Sesquiterpenoids from Commiphora myrrh Resin. Molecules 2020, 25, 1318. [Google Scholar] [CrossRef] [PubMed]
- Nasr, F.A.; Noman, O.M.; Alqahtani, A.S.; Qamar, W.; Ahamad, S.R.; Al-Mishari, A.A.; Alyhya, N.; Farooq, M. Phytochemical constituents and anticancer activities of Tarchonanthus camphoratus essential oils grown in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Bremer, E.; Van Dam, G.; Kroesen, B.J.; de Leij, L.; Helfrich, W. Targeted induction of apoptosis for cancer therapy: Current progress and prospects. Trends Mol. Med. 2006, 12, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljević, S.; Jelača, S.; Zengin, G.; Mimica-Dukić, N.; Berežni, S.; Miljić, M.; Stevanović, Z.D. Alchemilla vulgaris agg.(Lady’s mantle) from central Balkan: Antioxidant, anticancer and enzyme inhibition properties. RSC Adv. 2019, 9, 37474–37483. [Google Scholar] [CrossRef] [PubMed]
- Moqidem, Y. Evaluation of the Anticancer Potential of Alchemilla vulgaris Extract Against Human Neuroblastoma Cells; The American University in Cairo, AUC Knowledge Fountain: Cairo, Egypt, 2021. [Google Scholar]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Yadav, A.S.; Gothalwal, R. Assessment of total phenolic, flavonoid content and in vitro antioxidant properties of Alchemillia vulgaris (lady’s mantle). J. Adv. Sci. Res. 2021, 12, 205–209. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, P.D.; Lopez-Hernandez, F.J.; Perez-Barriocanal, F.; Morales, A.I.; Lopez-Novoa, J.M. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol. Dial. Transplant. 2011, 26, 3484–3495. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.K.; Raina, R.; Prawez, S.; Sultana, M.; Singh, M.; Kumar, P. Protective mechanisms of quercetin on cisplatin induced oxidative damage in hepatic tissue of wistar rats. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1399–1407. [Google Scholar] [CrossRef]









| Gene | Primer |
|---|---|
| BAX | F: 5’-ATGGACGGGTCCGGGGAG-3’ |
| R: 5’-ATCCAGCCCAACAGCCGC-3’ | |
| BCL2 | F: 5’-AAG CCG GCG ACGACT TCT-3’ |
| R: 5’-GGT GCC GGT TCA GGTACT CA-3’ | |
| P53 | F: 5’-ATGTTTTGCCAACTGGCCAAG -3’ |
| R: 5’-TGAGCAGCGCTCATGGTG-3’ |
| No | Compound | RT * min | Concentration μg/mL | µg/g Dry Extract | µg/g Root Powder |
|---|---|---|---|---|---|
| 1 | Unknown | 2.8 | NA ** | NA | NA |
| 2 | Unknown | 3.4 | NA | NA | NA |
| 3 | Syringenic acid | 5.1 | 5.33 | 106.6 | 7.6 |
| 4 | Cinnamic acid | 7.0 | 18.05 | 361 | 25.8 |
| 5 | Caffeic acid | 8.0 | 17.26 | 345.2 | 24.7 |
| 6 | Gallic acid | 9.7 | 4.79 | 95.8 | 6.8 |
| 7 | Salicylic acid | 12.3 | 9.56 | 191.2 | 13.7 |
| 8 | Ellagic acid | 12.9 | 8.49 | 169.8 | 12.1 |
| 8 | Unknown | 15.0 | NA | NA | NA |
| 10 | Protocatchuic acid | 15.5 | 2.21 | 44.2 | 3.2 |
| 11 | Unknown | 15.7 | NA | NA | NA |
| 12 | Unknown | 16.0 | NA | NA | NA |
| No | Compound | RT * min | Concentration μg/mL | µg/g Dry Extract | µg/g Root Powder |
|---|---|---|---|---|---|
| 1 | Unknown | 2.8 | NA ** | NA | NA |
| 2 | Naringin | 4.4 | 6.33 | 126.6 | 9.1 |
| 3 | Rutin | 5.2 | 5.14 | 102.8 | 7.4 |
| 4 | Querestin | 7.0 | 10.28 | 205.6 | 14.7 |
| 5 | Kampferol | 7.9 | 4.23 | 84.6 | 6.0 |
| 6 | Luteolin | 9.1 | 3.88 | 77.6 | 5.5 |
| 7 | Hisperdin | 10.0 | 11.56 | 231.2 | 16.5 |
| 8 | Catechin | 12.0 | 7.69 | 153.8 | 11.0 |
| Extract Concentrations (µg/mL) | Inhibition Zone (mm) | Growth Inhibition (%) | ||||
|---|---|---|---|---|---|---|
| Seratia | Acinetobacter | Agrobacterium | Seratia | Acinetobacter | Agrobacterium | |
| 15.6 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 0.00 | 0.00 | 0.00 |
| 31.3 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 0.00 | 0.00 | 0.00 |
| 62.5 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 0.00 | 0.00 | 0.00 |
| 125 | 7.67 ± 0.47 | 8.00 ± 0.00 | 8.00 ± 0.34 | 27.78 | 33.33 | 33.33 |
| 250 | 8.00 ± 0.82 | 8.33 ± 0.94 | 7.67 ± 0.46 | 33.33 | 38.89 | 27.78 |
| 500 | 10.33 ± 0.46 | 11.33 ± 0.47 | 9.33 ± 0.44 | 72.22 | 88.89 | 55.56 |
| 1000 | 11.00 ± 0.04 | 12.33 ± 0.40 | 11.00 ± 0.82 | 83.33 | 105.56 | 83.33 |
| Negative control | 6.0 ± 0.00 | 6.00 ± 0.00 | 6.0 ± 0.00 | 0.00 | 0.00 | 0.00 |
| Amoxicillin (62.5 ppm) | 41.3 0.40 | 38.33 ±1.25 | 37.0 ± 84 | 588.89 | 538.89 | 516.67 |
| LSD (0.05) | 0.81 | 1.19 | 0.93 | - | - | - |
| Extract Concentrations (µg/mL) | Mycelial Growth (mm) | Growth Inhibition (%) | ||||
|---|---|---|---|---|---|---|
| Rhizoctonia | Penicillium | Fusarium | Rhizoctonia | Penicillium | Fusarium | |
| 15.6 | 8.23 ± 0.05 | 8.30 ± 0.08 | 6.17 ± 0.12 | 8.52 | 7.78 | 31.48 |
| 31.3 | 8.23 ± 0.05 | 8.10 ± 0.08 | 5.70 ± 0.08 | 8.52 | 10.00 | 36.67 |
| 62.5 | 7.87 ± 0.09 | 7.83 ± 0.24 | 5.63 ± 0.09 | 12.59 | 12.96 | 37.41 |
| 125 | 7.87 ± 0.05 | 7.60 ±0.28 | 5.50 ± 0.14 | 12.59 | 15.56 | 38.89 |
| 250 | 7.37 ± 0.07 | 7.30 ±0.18 | 5.17 ± 0.12 | 18.15 | 18.89 | 42.59 |
| 500 | 7.37 ± 0.12 | 7.30 ±0.16 | 5.23 ± 0.09 | 18.15 | 18.89 | 41.85 |
| 1000 | 7.00 ± 0.22 | 6.97 ± 0.05 | 4.90 ± 0.08 | 22.22 | 22.59 | 45.56 |
| Negative control | 9.0 ± 0.00 | 9.0 ± 0.00 | 9.0 ± 0.00 | 0.00 | 0.00 | 0.00 |
| Hymexazol (1000 ppm) | 1.8 ± 0.24 | 2.9 ± 0.69 | 1.6 ± 0.12 | 79.63 | 68.15 | 81.85 |
| LSD (0.05) | 0.26 | 0.58 | 0.22 | - | - | - |
| IC50 (µg/mL) | Selectivity Index | ||||||
|---|---|---|---|---|---|---|---|
| Vero | MCF-7 | PC-3 | Caco-2 | MCF-7 | PC-3 | Caco-2 | |
| A. vulgaris root extract | 592.47 | 92.25 | 88.60 | 110.51 | 6.42 | 6.69 | 5.36 |
| Doxorubicin | 35.09 | 5.40 | 34.11 | 35.09 | 6.50 | 1.03 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, O.H.M.; Abo-Elyousr, K.A.M.; Asiry, K.A.; Alhakamy, N.A.; Mousa, M.A.A. Phytochemical Characterization, Antimicrobial Activity and In Vitro Antiproliferative Potential of Alchemilla vulgaris Auct Root Extract against Prostate (PC-3), Breast (MCF-7) and Colorectal Adenocarcinoma (Caco-2) Cancer Cell Lines. Plants 2022, 11, 2140. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11162140
Ibrahim OHM, Abo-Elyousr KAM, Asiry KA, Alhakamy NA, Mousa MAA. Phytochemical Characterization, Antimicrobial Activity and In Vitro Antiproliferative Potential of Alchemilla vulgaris Auct Root Extract against Prostate (PC-3), Breast (MCF-7) and Colorectal Adenocarcinoma (Caco-2) Cancer Cell Lines. Plants. 2022; 11(16):2140. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11162140
Chicago/Turabian StyleIbrahim, Omer H. M., Kamal A. M. Abo-Elyousr, Khalid A. Asiry, Nabil A. Alhakamy, and Magdi A. A. Mousa. 2022. "Phytochemical Characterization, Antimicrobial Activity and In Vitro Antiproliferative Potential of Alchemilla vulgaris Auct Root Extract against Prostate (PC-3), Breast (MCF-7) and Colorectal Adenocarcinoma (Caco-2) Cancer Cell Lines" Plants 11, no. 16: 2140. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11162140